GDMT, or Guideline-Directed Medical Therapy, is commonly used as shorthand to refer to the four pillars of medications used in HFrEF, or Heart Failure with reduced Ejection Fraction. I am referencing the 2022 AHA/ACC/HFSA guideline document in Circulation to review these clinical trials.
The four pillars of GDMT include:
ACEi/ARB/ARNi
Beta Blockers
Mineralocorticoid Receptor Antagonists (MRA)
SGLT2 inhibitors
We will review these drug classes and the specific trials cited in the guidelines that justify their use in HFrEF. A previous post critiquing the preferred status of Entresto outlines the basics categories of heart failure as measured by ejection fraction. If you are interested in that refresher, read the first section in this post.
Pillar 1: ACEi/ARB/ARNi
Background: There are three types of medications in this category.
ACEi = Angiotensin-Converting Enzyme Inhibitors
These drugs have names ending in “-pril”, such as lisinopril, captopril, enalapril…
ARB = Angiotensin II Receptor Blockers
These drugs have names ending in “-sartan”, such as valsartan, losartan, candesartan…
ARNi = ARB + Neprilysin Inhibitor
There is only one ARNi approved by the FDA for treatment of heart failure. sacubitril-valsartan.
I will re-use my segment in a previous post about Entresto to review the basic physiology:
RAAS and its pharmaceutical targets
The Renin-Angiotensin-Aldosterone-System (RAAS) functions to increase blood pressure and intravascular volume. If you start from the box in the top left of this picture, it is a good overview:
ACE inhibitors prevent angiotensin I from being converted to angiotensin II. ARBs prevent angiotensin II from binding to receptors. When we think about the only ARNi in use, sacubitril-valsartan, there are two things going on. The valsartan component is an ARB, sacubitril is a neprilysin inhibitor.
Neprilysin is an enzyme that breaks down natriuretic peptides, bradykinin, adrenomedullin, and angiotensin II. By inhibiting neprilysin, sacubitril increases the levels of these substances.
Benefits of neprilysin inhibition: Vasodilation, decreased sympathetic nervous system (SNS) tone, natriuresis/diuresis
Downside: Increased angiotensin II.
Solution: Combination of neprilysin inhibitor (sacubitril) and an ARB (valsartan)
The general theme here is that these three classes of medications function to block RAAS. This reduces blood pressure and peripheral vasoconstriction, decreasing strain on the heart. There are other effects, and they vary based on which agent is used, but this is a basic overview. I consider this the first pillar of GDMT, as ACEi specifically were widely adopted first. For brevity, I will only cover one trial for each drug class in this category of GDMT.
Clinical Trials of ACEi/ARB/ARNi
First a quick review of trials, then I will provide some brief commentary.
Note: For both ACEi and ARBs, there are a huge number of possible trials to choose from. I chose a trial that the guideline I am reviewing cited for ACEi, but for ARBs this was not possible. The reason I end up choosing ELITE II is that it came across my radar and fits into the chronic HFrEF category.
ACE inhibitors
SOLVD Trial (Enalapril)
“Effect of Enalapril on Survival in Patients with Reduced Left Ventricular Ejection Fractions and Congestive Heart Failure”
Published in 1991 in NEJM
Criteria and Outcomes
2,569 patients with stable congestive HF and EF <0.35 were enrolled
Run-in phase started with 2.5 mg enalapril twice daily lasting 2-7 days. Patients then placed on placebo for 14-17 days to ensure compliance and stability.
Randomization to 2.5 or 5mg twice daily of treatment vs. placebo was followed by up titration to a maximum of 10mg twice daily as tolerated
1285 patients were randomized to enalapril, 1284 to placebo
Primary outcome was all-cause mortality
Characteristics
90% of pts were NYHA class II or III. Follow-up average was 41.4 months
Mean age of pts was 61, mean EF was 25%, HR 80bpm, SBP 125, DBP 77, pts were mostly male 80%
Background therapy consisted of diuretics 85%, digitalis ~67%, and vasodilators 50%
Results
There was crossover into the treatment arm, and the table below I think summarizes the important outcomes and neatly displays this crossover, here was the description from the study:
“In patients with worsening symptoms of congestive heart failure, an increase in the dose of diuretic agents or the addition of other vasodilators was generally recommended as the first step. If the patient remained symptomatic despite maximal therapy with such medications, open-label treatment with an angiotensin-converting–enzyme inhibitor was allowed, and the blinded medication was discontinued. However, all randomized patients were retained in the analysis.”
The benefit seen in this trial was concentrated among patients who were hospitalized for heart failure at least once. The study authors suggest this indicates the benefit of enalapril was primarily via attenuation of progression of heart failure. There was no difference in deaths from arrhythmia without worsening heart failure. This is one issue we will see later which beta blockers seem to address.
ARBs
Valsartan
Using the 2022 AHA/ACC/HFSA guideline I was unable to find a ARB vs. ACEi straight up comparison in stable HFrEF. Briefly, The Valsartan Heart Failure Trial (VAL-HeFT), published in 2001 in NEJM showed that valsartan vs. placebo with background ACEi use of >90% did not affect mortality, though it did reduce the risk of hospitalization for heart failure.
Subgroup analysis of this study showed mortality benefit of valsartan among patients who were not on background ACEi or beta blockade. Patients on both ACEi and beta blockers may have been harmed by the addition of valsartan. Background beta blocker use was ~35% in VAL-HeFT.
The Valsartan in Acute Myocardial Infarction (VALIANT) trial, published in 2003 in NEJM, tested the use of valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. They randomized roughly 4900 patients into each of the three groups, within 10 days of myocardial infarction, and followed them for a median duration of two years. This trial showed no meaningful difference between valsartan, captopril, or both in terms of mortality or hospitalizations for heart failure.
The groups receiving valsartan had a higher rate of hypotension and renal adverse events. The groups receiving captopril had higher rates of cough, rash, and angioedema, though the angioedema result was directional and not statistically significant.
Losartan
Although it wasn’t referenced in the guideline, we will briefly review the ELITE II study to satisfy the direct comparison of an ARB with an ACEi in stable HFrEF.
ELITE II Trial (Losartan)
Published in the Lancet in 2000.
Criteria and Outcomes
Randomized 3152 patients 60 years+ with NYHA class II-IV heart failure and EF of 40% or less
1578 patients assigned losartan titrated to 50mg once daily
1574 patients assigned captopril titrated to 50mg three times d
Run in period of 1-28 days of single-blind placebo to ensure stability and adherence
Primary end point was all-cause mortality
Secondary end points were sudden death or resuscitated arrest
Median follow up was 555 days
Characteristics
Mean age 71, mostly men (70%), mostly NYHA class II or III (95% combined), mean EF 31%, HR 75bpm, SBP 134, DBP 78
Background therapy of diuretics (78%), cardiac glycoside (50%) and aspirin/salicylates (60%). Roughly 22% of patients also on beta blockers.
Results
As you can see, losartan was not superior to captopril. If anything, captopril seems directionally to have had superior outcomes, though not statistically significant. Of note, subgroup analysis suggested that among patients on a beta blocker, survival was increased in the captopril group, relative to losartan. Losartan was better tolerated, however.
Here is the tolerability figure, which happens to be a comically bad figure. Not sure if it just looks like this because I am viewing the pdf, but just know losartan is left, captopril is right.
Thoughts on ELITE II: This trial followed the ELITE trial, which can be summed up by quoting the interpretation from its abstract:
“In this study of elderly heart-failure patients, treatment with losartan was associated with an unexpected lower mortality than that found with captopril. Although there was no difference in renal dysfunction, losartan was generally better tolerated than captopril and fewer patients discontinued losartan therapy. A further trial, evaluating the effects of losartan and captopril on mortality and morbidity in a larger number of patients with heart failure, is in progress.”
The only reason I mention this is because it is a great example of why smaller trials and secondary end points should be interpreted with caution. ELITE sought to compare tolerability of losartan versus captopril in elderly patients with HFrEF. They randomized just over 700 patients. A secondary endpoint suggested a significant mortality benefit of losartan vs captopril, but this was hypothesis-generating. ELITE II, a much larger trial (roughly 4x), showed that this result was likely spurious.
The takeaway from the authors in ELITE II was that ACEi should remain first-line, with losartan as a reasonable alternative for intolerant patients.
Note: I should mention that I am aware of the CHARM trials, which seemed to show that candesartan, another ARB, demonstrated mortality and morbidity benefit both in the presence and absence of background ACEi. These are the CHARM-Added and CHARM-Alternative trials. This is turning into a rabbit hole and we are in the first pillar, so I will move on. I think it is fair to say that ACEi and ARBs provide similar benefit in HFrEF, but generalization by class may be risky, and individual products should be assessed based on clinical trial data. ARBs tend to reduce cough and angioedema relative to ACEi, while possibly increasing hypotension and renal events.
ARNi
Sacubitril-valsartan
There is only one ARNi, sacubitril-valsartan, brand name Entresto. It has preferred status in HFrEF, which is to say it is recommended in favor of ACEi or ARBs. I have a dedicated post that addresses my qualms surrounding the evidence behind this decision. Link here.
This will end up being a huge post, so I will not include discussion of those trials here, please use that link and scroll down to the clinical trials if you want the details. The TLDR is that I don’t believe it has earned that preferred status.
Someone once made a great point that I would like to bring up. Look at all of the ACEi that we have, look at all of the ARBS. It seems like there are a dozen “-pril” and a dozen “-sartan” drugs. There is one ARNi. If this drug class is so effective, where are the competitors? We love to talk about the mechanism of action of the neprilysin inhibitor in combination with ARB, but where are the other drugs in this class?
To be clear, I am not saying any specific drug is better than or even as good as Entresto. I’m saying I simply don’t know, because the proper clinical trials were never run. Okay, let’s move on to beta blockers.
Pillar 2: Beta Blockers
Physiology Basics: Adrenergic receptors divide into alpha and beta receptors. Here is a useful diagram showing their function.
For our purposes, we can focus on beta-1, beta-2, and alpha-1 receptors. These receptors have well-characterized distribution within the body. Here is a simplified way to think about each one:
Beta-1 Receptors: We will focus on the heart specifically, where beta 1 receptor activation leads to an increase in heart rate and contractility. More beta-1 tone—> more rapid and stronger pumping of the heart.
Beta-2 Receptors: Think of these as broadly distributed through the vasculature. There are many other important locations and functions, as the diagram above suggests, but we can think of beta-2 activation leading to peripheral vasodilation, which in turn lowers blood pressure.
Alpha-1 Receptors: For our purposes, I think of these as having the opposite effect of beta-2. Alpha-1 receptors lead to peripheral vasoconstriction, which increases blood pressure.
Beta blockers can be divided into non-selective and cardio-selective beta blockers. Cardio-selective means they exert their effects predominantly through blockade of beta-1 receptors, while non-selective block both types of beta receptors. Two of the three beta blockers recommended for use in HFrEF are cardio-selective. These include metoprolol and bisoprolol. Carvedilol, the third approved beta blocker, is non-selective, with the additional function of blocking alpha-1 receptors.
Blocking the beta-1 receptor reduces the oxygen demand on a dysfunctional heart, decreasing the stress on the muscle and improving outcomes. Lowering the heart rate by this same mechanism can provide anti-arrhythmic protection. We will see in these trials that sudden deaths were dramatically reduced. Blocking alpha-1, as in the case of carvedilol, leads to additional reduction in blood pressure.
Note: Beta-1 blockade also reduces renin release, the first step in the RAAS cascade.
Before we jump into the studies, final review:
Metoprolol and Bisoprolol block beta-1 receptors.
Carvedilol blocks beta-1, beta-2, and alpha-1 receptors.
Clinical Studies of Beta Blockers
We will cover the basics of the three big trials here, and I will hold comments until after.
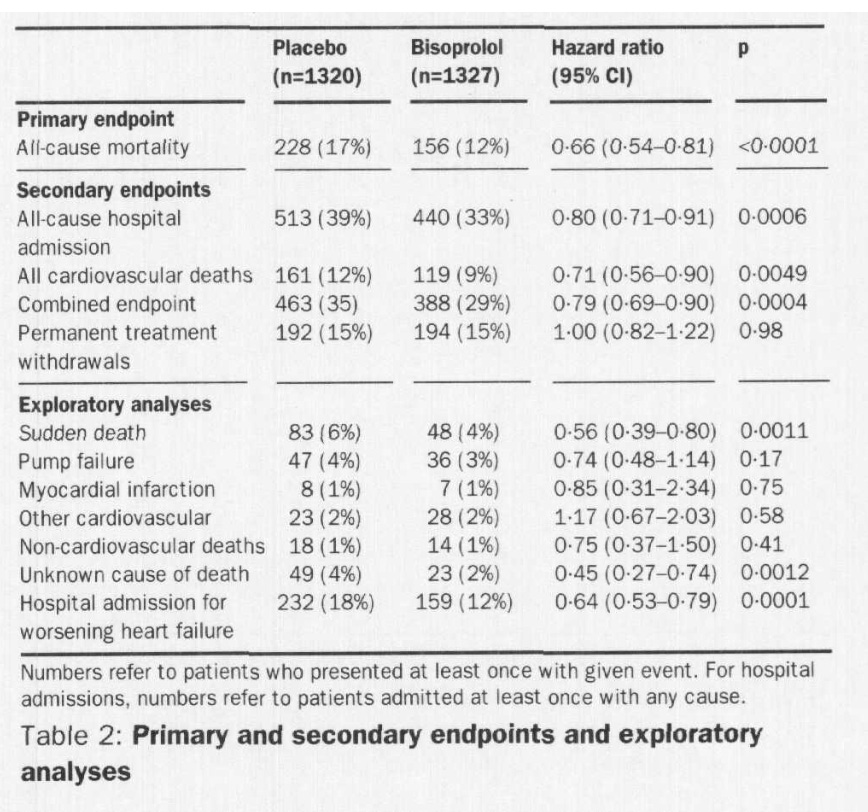
CIBIS-II Trial (Bisoprolol)
“The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial”
Published in 1999 in the Lancet.
10mg daily target dose Bisoprolol
Enrolled 2,647 patients, NYHA class III or IV, LVEF 35% or less receiving diuretics and ACEi.
1,327 randomized to bisoprolol (initial dose 1.25mg), 1,320 assigned placebo.
Follow-up was for a mean of 1.3 years, analysis was by intention to treat.
Patients were stable with chronic heart failure, mean age 61, mostly men (80%), average EF 27%, HR 80bpm, SBP 130, DBP 80.
Primary endpoint was all-cause mortality
The study was stopped early for efficacy, endpoints shown below.
MERIT-HF Trial (Metoprolol)
“Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure”
Published in 1999 in the Lancet.
200mg daily target dose of Metoprolol CR/XL (Controlled release/ extended release)
Enrolled 3,991 patients with symptomatic heart failure for 3+ months receiving optimal therapy (90% of pts on diuretics + ACEi). Patients had LVEF of 0.40 or lower within 3 months of enrollment.
Patients were mostly men (~77%), average age 64, average EF of 0.28, HR 82bpm, SBP 130, DBP 78.
There was a single-blind, two week placebo run-in period, further assuring stability in these patients.
1,990 patients assigned metoprolol CR/XL, 2,001 assigned placebo.
Starting dose was 12.5mg or 25mg once daily. After two weeks dose was increased to 50mg once daily, then two weeks later 100mg once daily, and finally up to the target dose of 200mg once daily.
Two primary endpoints included all-cause mortality (ACM) and ACM + all-cause admission to hospital. Analysis was by intention to treat.
The study was stopped early on recommendation of the independent safety committee. At the time of stoppage the mean follow-up time was one year.
145 patients in the metoprolol group had died, versus 217 patients in the placebo group. There were 128 cardiovascular deaths in the treatment group, versus 203 in placebo.
Study drug discontinuation was similar between metoprolol and placebo (~14%). 64% of patients on metoprolol had reached the target dose of 200mg daily by the end of the study. 87% of patients were receiving 100mg or more.
6 months after randomization, HR had decreased by 14bpm in metoprolol group, vs 3bpm in placebo.
Summary: “Once-daily metoprolol CR/XL added to optimum standard treatment with primarily ACE inhibitors and diuretics lessened all-cause mortality by 34% in clinically stable patients with symptomatic chronic heart failure and lowered ejection fraction in NYHA functional classes II-IV. Therefore, treatment of 27 patients with metoprolol CR/XL for 1 year can prevent one death.”
COPERNICUS Trial (Carvedilol)
“Effect of Carvedilol on Survival in Severe Chronic Heart Failure”
Published in 2001 in NEJM
2,289 patients with EF <25% and presence of symptoms of heart failure at rest or on minimal exertion, who were clinically euvolemic.
1133 patients randomly assigned placebo, 1156 assigned carvedilol
Mean follow up at time of trial stoppage was 10.4 months
Patients excluded if they required intensive care, had marked fluid retention, or were receiving IV vasodilators or positive inotropic drugs
Initial dose was 3.125 mg carvedilol or placebo twice daily for two weeks, which was increased at two week intervals to 6.25, 12.5 and to target of 25mg twice daily, as tolerated
Primary end point was all cause mortality, analysis was by intention to treat
Patients had mean age 63, mostly male (80%), average LVEF of 20%, HR 83bpm, SBP 123, DBP 76. Almost all patients were on both diuretics (99%) and ACEi (97%), 20% of patients were receiving MRA (spironolactone)
“According to the intention-to-treat analysis, 190 patients in the placebo group died and 130 patients in the carvedilol group died; this difference reflected a 35 percent decrease in the risk of death with carvedilol…..the cumulative risk of death at one year was 18.5 percent in the placebo group and 11.4 percent in the carvedilol group.”
Brief thoughts on beta blocker trials: I think the results largely speak for themselves. All of these trials showed impressive benefits in meaningful outcome measures, most importantly all-cause mortality. It’s important to not lose sight of the fact that all of these trials, even COPERNICUS, were in stable patients. Stable HFrEF is obviously very different from a patient immediately s/p revascularization following acute myocardial infarction.
I found it interesting to note the relatively high use of digitalis in this era, which was over 50% in all three of the trials. In any case, these studies together strongly recommend the use of one of these agents in stable HFrEF. It’s impressive to see this additional benefit on top of ACE inhibition.
Pillar 3: Mineralocorticoid Receptor Antagonists (MRAs)
Physiology Basics: We can keep things simple here, zooming in on the final part of the RAAS pathway we discussed for ACEi/ARB/ARNi. Aldosterone, the major mineralocorticoid hormone, is produced in the adrenal gland. It causes the kidney to retain sodium and excrete potassium. This leads to water retention and increased blood pressure.
Blocking the receptor that aldosterone uses with MRAs leads to less sodium and water retention, but more potassium retention. As always, there are other mechanisms involved, and these receptors are found not only in the kidney but in the heart and blood vessels. In heart failure this pathway is over-activated and the mineralocorticoid receptor is an attractive drug target to improve outcomes in HFrEF.
One final important concept is selective versus nonselective MRAs. The basic idea is that non-selective MRAs like spironolactone have meaningful anti-androgen effects, in addition to the mineralocorticoid blockade. We will see this play out in the side effects reported when men are given spironolactone. Gynecomastia can occur, which is an increase in breast tissue in men.
Eplerenone is a selective MRA, which is to say its affinity for the mineralocorticoid receptor is far more specific, and you don’t get the anti-androgen side effects. The table below demonstrates the relative affinity of these drugs for different receptors. The lower the number, the more inhibition of the receptor.
RALES Trial (Spironolactone)
“Randomized Aldactone Evaluation Study” (RALES)
Published 1999 in NEJM
Note: Aldactone is the brand name for spironolactone. Not a fan of using brand names in trials but I guess it makes a decent acronym.
1663 patients were randomized, 841 assigned placebo, 822 assigned spironolactone
This was a study of severe heart failure, eligible patients had NYHA class IV heart failure within 6 months of enrollment and were NYHA class III or IV at time of enrollment and had LVEF <35%
Mean age was 65, mostly male (73%), HR 81bpm, SBP 122, DBP 75, LVEF 25
Background therapy consisted of loop diuretics (100%), ACEi (94%), digitalis (~74%), beta blockers (10%)
Initial randomization was to 25 mg daily spironolactone or placebo, after 8 weeks, dose increase to 50mg once daily was allowed if patients had signs/symptoms of progression of heart failure and no evidence of hyperkalemia
The trial was stopped early for benefit with a mean follow up of 24 months
Primary end point was all-cause mortality
Secondary end points included cardiac death and cardiac hospitalization
Analysis was by intention to treat
Results:
The spironolactone group had an increase in median serum potassium concentration of 0.3 mmol/L. 10% of men in the spironolactone group reported gynecomastia or breast pain, versus just 1% of men in the placebo group.
Of note, there was no meaningful change observed in hemodynamics in this study from spironolactone. The authors suggest the benefit realized in this case may be attributable to the direct cardiac and vascular protective effects of spironolactone.
EMPHASIS-HF Trial (Eplerenone)
“Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms”
Published 2011 in NEJM
Side Note: This study followed an earlier trial of Eplerenone, published in 2003 in NEJM “Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS)”. That study established survival benefit of Eplerenone post-MI. Now back to EMPHASIS-HF.
Eligible patients were at least 55 years of age, NYHA functional class II, with EF 30% or less, or, if 30-35%, had a QRS duration of >130msec on ECG.
Randomization was to occur within 6 months of hospitalization for cardiovascular reason. Patients without hospitalization but with elevated BNP could also be enrolled.
Exclusion criteria: NYHA class III or IV, acute myocardial infarction, potassium >5mmol/L, eGFR less than 30
Eplerenone was started at 25mg daily and increased after 4 weeks to 50mg once daily. Dose reductions were planned if potassium was elevated beyond 5.5 at follow up appointments.
Primary outcome: death from cardiovascular causes or a first hospitalization for heart failure.
1364 patients randomly assigned eplerenone, 1373 placebo.
Mean age was 69, 22% female, HR 72bpm, SBP 124, DBP 75, LVEF 26%
Background therapy: ~93% patients on ACEi, ARB, or both, 86% on beta blocker, 26% on digitalis, 85% on diuretic
Results
Adverse events: Hyperkalemia was more common in the eplerenone group, of note, there was no difference in gynecomastia or other breast disorders.
Brief thoughts on MRA trials
The trials we reviewed both show meaningful benefit from MRAs. The populations were quite different, given the severity of heart failure studied in RALES, versus EMPHASIS-HF. Eplerenone, as a selective MRA, seems to avoid the anti-androgen side effect of gynecomastia.
We can clearly see the implementation of beta blockers in HFrEF in these trials. RALES was published in 1999, around the same time that CIBIS-II, MERIT-HF, and COPERNICUS were published. Background beta blocker therapy in RALES was just 10%, versus 86% in EMPHASIS-HF, which was published in 2011. We also see far less digitalis, though I am not sure to what degree that reflects the shift away from these products vs. milder heart failure.
Pillar 4: Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors
Physiology Basics: The proximal convoluted tubule (PCT) of the nephron is the site of bulk absorption of filtered glucose and sodium. SGLT2 proteins facilitate this absorption, transporting both sodium and glucose across the apical membrane. When this action is reduced by SGLT2 inhibitors, glucose can be renally cleared, lowering blood glucose.
This is a simplified explanation but it is not my intention to get into the weeds with proposed mechanisms of action for SGLT2 inhibitors. For now I will just mention that effects on RAAS inhibition, reductions in preload/afterload, and ketone metabolism are a few other things that are often brought up. Lowering blood glucose is an attractive drug effect, especially in the world of type 2 diabetes mellitus (T2DM). The original FDA approvals for drugs in this class were for the treatment of T2DM. Unexpected cardioprotective effects were observed in those trials (eg. reductions in hospitalizations for heart failure), and the rest is history.
The two drugs we will discuss are dapagliflozin and empagliflozin, both of which are FDA approved for T2DM, HFrEF, as well as chronic kidney disease (CKD). These are really impressive drugs with a lot of trial data, but we will focus on the two big studies relevant for our topic.
DAPA-HF Trial (Dapagliflozin)
“Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction”
Published 2019, NEJM
Criteria and Outcomes
Eligible patients were adults with an EF of 40% or less and NYHA class II, III, or IV. Pts had to have NT-proBNP of at least 600 pg/mL, or at least 400 pg/mL if hospitalized for HF in last year.
Patients had to be on standard background therapy, including RAAS inhibition and beta blockade. MRA use was encouraged.
Patients were excluded if eGFR was less than 30, symptoms of hypotension of SBP <95mmHg, T1DM.
Pts randomized to dapagliflozin (10mg once daily) or placebo. Randomization was stratified based on diagnosis of T2DM
Primary outcome was composite of worsening HF or death from cardiovascular causes. Key secondary outcome was composite of hospitalization for HF or cardiovascular death.
Analysis was intention to treat, median duration of follow-up was 18.2 months
Characteristics
Mean age was 66 years, just under a quarter of patients were women, mean BMI 28
Two thirds of pts were NYHA II, one third III, ~1% NYHA IV
Mean SBP 122, HR 71, LVEF 31%, NT-proBNP ~1435
Slight majority of pts with ischemic HF, almost half with history of hospitalization for HF, 41% with diabetes mellitus, just over a third with afib
Mean eGFR 66, 40% of pts in both groups with eGFR <60
Background Meds
93% on Diuretic
56% ACEi, ~27% ARB, 10% ARNI
96% on beta blocker
70% on MRA
18% on digitalis
Half of pts on biguanide, about a quarter of pts on insulin
Results
Primary outcome occurred in 16.3% of patients in treatment group, 21.2% in placebo group. Hazard ratio 0.74, 95% CI 0.65-0.85.
Hospitalizations for HF: 9.7% of treatment, 13.4% of placebo
Death from CV causes: 9.6% of treatment, 11.5% of placebo
The key secondary outcome was basically identical to the primary outcome
All cause mortality: 11.6% in treatment, 13.9% in placebo
Safety events were similar between groups, there were 3 diabetic ketoacidosis events in the treatment group (0.1% of patients), a rare adverse event but one that is important to be aware of for patients on these medications. We will not get into euglycemic DKA in this post but it is an interesting phenomenon.
EMPEROR-Reduced Trial (Empagliflozin)
“Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure”
Published 2020, NEJM
Criteria and Outcomes
Eligible patients were adults with CHF with LVEF <40%.
Goal was to enroll particularly high risk patients
Pts either had HF hospitalization in last 12 months or elevated NTproBNP ( >600pg/mL for EF <30%, >1000pg/mL for EF 31-35%, >2500pg/mL in EF 36-40%)
These thresholds were doubled for patients with afib
Pts randomly assigned 1:1 to either empagliflozin or placebo
Randomization stratified by region, diabetes status, and eGFR at screening
Primary outcome was composite of adjudicated cardiovascular death or hospitalization for heart failure, time to first event
Secondary outcomes:
All adjudicated hospitalizations for heart failure (first and recurrent events)
Rate of decline in eGFR
Analysis was by intention to treat
Characteristics
7220 patients screened, 3730 randomly assigned
Vast majority of screening failure was due to not meeting NTproBNP criteria
Median follow up was 16 months
Mean age 67, 24% female, 75% NYHA II, 24% NYHA III
Mean BMI 28, HR 71, SBP 122, LVEF 27%, NT-proBNP median ~1900
~72% of pts with LVEF<30%
Half of pts with ischemic HF, 30% with hospitalization for HF in last year, 36% with afib, 50% with DM, 72% HTN, mean eGFR 62, 48% of pts with eGFR <60
Meds:
RAAS inhibitor in 89% of pts (~19% of that with neprilysin inhibitor)
MRA in 70%
Beta blockade in 95%
Results
Primary outcome: 19.4% in treatment, 24.7% in placebo
Hazard ratio for CV death: 0.92 (95% CI, 0.75-1.12)
10.0% treatment, 10.8% placebo
Hazard ratio for first hospitalization for HF: 0.69 (95%CI 0.59-0.81)
13.2% treatment, 18.3% placebo
Secondary outcomes:
Total # of hospitalizations for HF was lower in empagliflozin group than placebo, 388 vs. 553 events (HR 0.70)
Rate of decline in eGFR was lower in empagliflozin group than placebo (-0.55 vs. -2.28) per year
Death from any cause: 13.4% empagliflozin, 14.2% placebo (nonsignificant HR)
Safety events were similar between the groups. There was a ~1% absolute risk increase of uncomplicated genital tract infections in the empagliflozin group.
Brief thoughts on DAPA-HF and EMPEROR-Reduced:
Both of these trials show a benefit with respect to hospitalizations for heart failure with SGLT2 inhibition. Dapagliflozin was more impressive with respect to CV mortality and all-cause mortality. Of note, the opposite was observed in CV outcomes in trials exclusively studying patients with T2DM. Empagliflozin (EMPA-REG OUTCOME) was more impressive with respect to cardiovascular and all-cause mortality than dapagliflozin (DECLARE-TIMI 58).
Patients were largely on appropriate background HF therapy, and the benefit seen in renal outcomes hints toward what would be a later approval for both of these drugs in CKD, based on the DAPA-CKD and EMPA-KIDNEY trials. These are impressive medications that seem to have earned their place as a pillar of current GDMT in HFrEF.
Concluding Thoughts: Apologies for the lengthy post, and congratulations if anyone made it to this point. I found it helpful and plan to use this post as a resource for future review when these trials inevitably fade from my memory. I am starting a rotation in the CCU soon, so hopefully I will have time to make less boring and more concise posts about whatever seems interesting in the world of cardiology.