The past few days I’ve been thinking about heart failure medications. Heart failure is common on the wards so it comes up quite often. One thing you’re certain to hear in these discussions is that patients should be on Entresto. In order to talk about the evidence behind this, it’s helpful to do a quick overview.
Heart Failure Basics
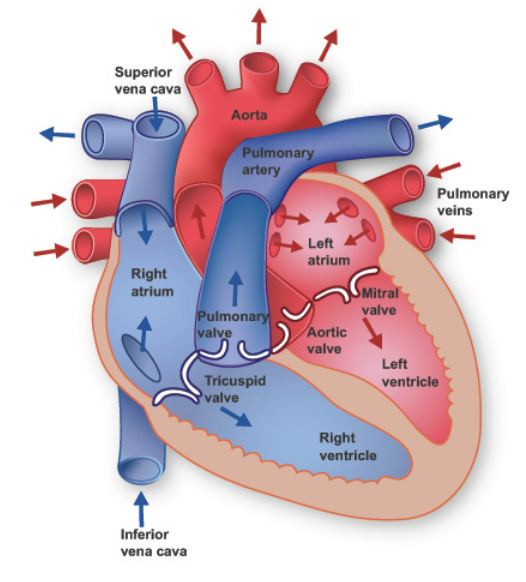
We will keep things simple and say that heart failure occurs when there is some cardiac problem that impairs the ability of the heart to pump blood. Let’s start by looking at the basic anatomy:
The left ventricle (LV) of the heart pumps blood through the aorta to the rest of the body. An important tool that is used to measure the function of the heart is an echocardiogram. This is an ultrasound, which is to say that it uses sound waves to capture dynamic images of the heart. Patients with heart failure can be categorized based on the function of their LV. The key measure is left ventricular ejection fraction (LVEF).
The LVEF tells us what percentage of blood in the left ventricle gets pushed out with each beat. The categories based on this measurement are as follows:
Heart failure with reduced ejection fraction (HFrEF): LVEF 40% or less
Heart failure with preserved ejection fraction (HFpEF): LVEF 50% or more
Heart failure with mid-range ejection fraction (HFmrEF): LVEF 41-49%
This will be relevant as we walk through the different trials in this post.
The etiologies of heart failure are numerous. They include ischemia, hypertension, infection, genetics, valvular disease, drug-induced (both recreational and medical), metabolic, arrhythmia, endocrine, etc.
Medications for HFrEF: Guideline-Directed Medical Therapy (GDMT)
There are four pillars of GDMT, but we will focus on only one of those pillars today. I will list which medication within these pillars are preferred in HFrEF:
ACEi/ARB/ARNI
ACEi: Angiotensin-Converting Enzyme inhibitors
ARB: Angiotensin II Receptor Blockers
ARNI: Angiotensin Receptor/Neprilysin inhibitor
Preferred: Sacubitril-valsartan (ARNI)
Alternatives: Lisinopril, Ramipril, Enalapril, Captopril, Trandolapril, Losartan, Candesartan, Valsartan
Note: “-pril” —> ACEi, “-sartan” —> ARB
Beta Blockers
Preferred: Carvedilol, Metoprolol Succinate, Bisoprolol
MRAs: Mineralocorticoid receptor antagonists
Preferred: Spironolactone or Eplerenone
SGLT2i: Sodium-glucose co-transporter 2 inhibitors
Preferred: Dapagliflozin or Empagliflozin
Alternative: Canagliflozin
We will focus on the evidence behind the preferred status of Sacubitril-valsartan. The brand name of Sacubitril-valsartan is Entresto, which I will use interchangeably. Before we look at the studies we should review the basics of how these drugs work.
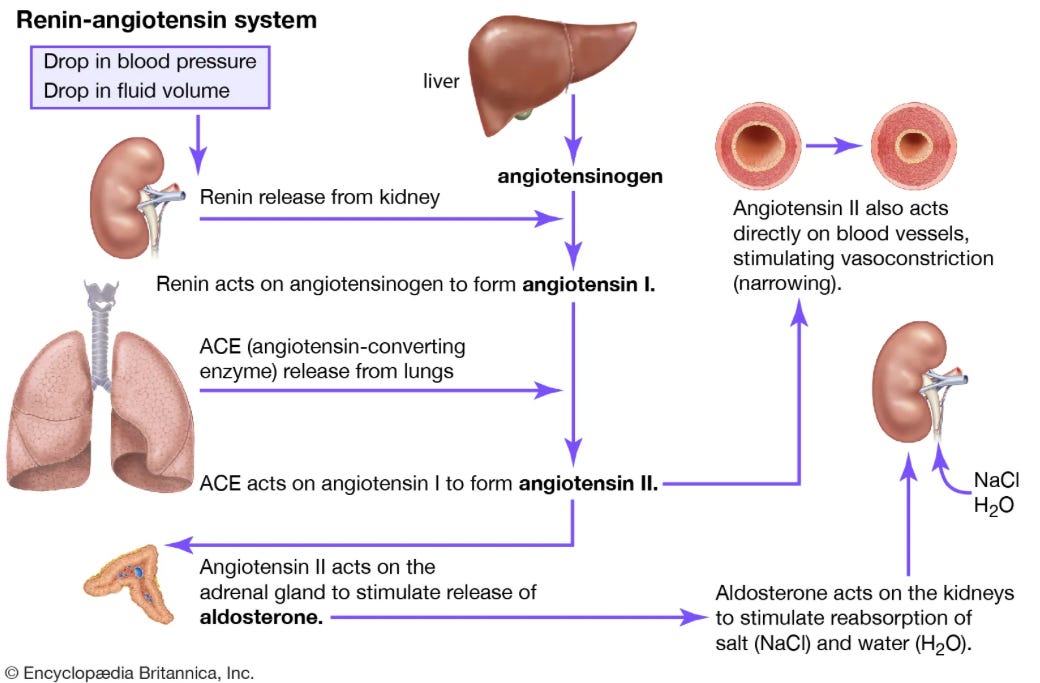
RAAS and its pharmaceutical targets
The Renin-Angiotensin-Aldosterone-System functions to increase blood pressure and intravascular volume. If you start from the box in the top left of this picture, it is a good overview:
This hopefully illustrates the effects of some of the medications listed earlier. ACE inhibitors prevent angiotensin I from being converted to angiotensin II. ARBs prevent angiotensin II from binding to receptors. When we think about Entresto, AKA sacubitril-valsartan, there are two things going on. The valsartan component is an ARB, sacubitril is a neprilysin inhibitor.
Neprilysin is an enzyme that breaks down natriuretic peptides, bradykinin, adrenomedullin, and angiotensin II. By inhibiting neprilysin, sacubitril increases the levels of these substances.
Benefits of neprilysin inhibition: Vasodilation, decreased sympathetic nervous system (SNS) tone, natriuresis/diuresis
Downside: Increased angiotensin II.
Solution: Combination of neprilysin inhibitor (sacubitril) and an ARB (valsartan)
Beautiful stuff, this is the kind of pathway and mechanism that people fall in love with. So now that we understand the medication, it makes sense that the addition of the neprilysin inhibitor could make Entresto a better drug than an ACEi or ARB alone. With that preamble behind us, let’s get to the trials.
Note: “BID” means twice daily.
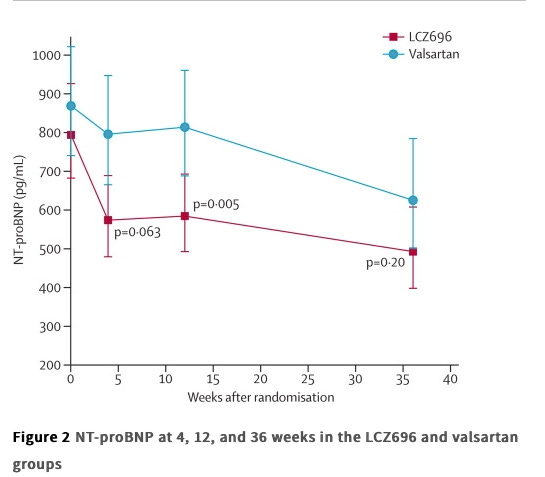
PARAMOUNT (2012, Lancet)
This was a phase II trial, total enrollment of just over 300 patients, so we will briefly mention what it showed. They took people with NYHA class II-III HF, LVEF 45% or higher, and NT-proBNP greater than 400pg/mL.
Arms: Entresto 200mg BID vs. valsartan 160mg BID
Outcomes: Change in NT-proBNP from baseline to 12 weeks.
Results:
The group that was randomized to Entresto had a reduction in NT-proBNP, relative to valsartan, though it didn't remain statistically significant at 36 weeks follow up.
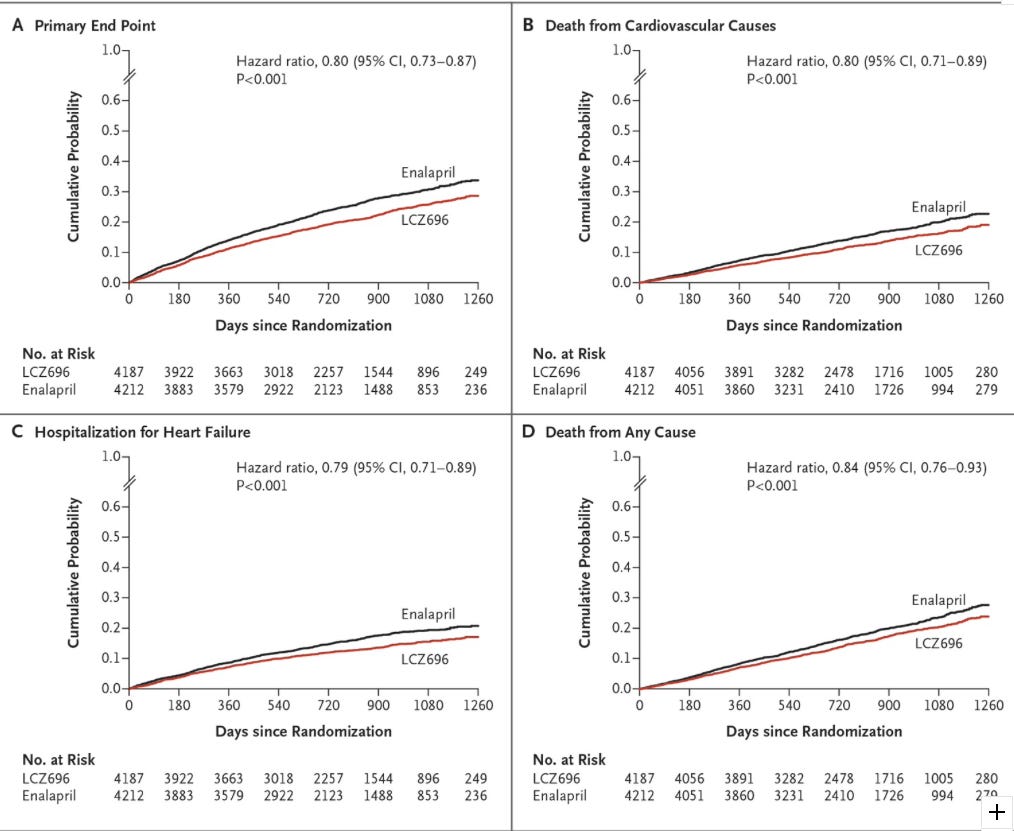
PARADIGM-HF (2014, NEJM)
Title: Angiotensin-Neprilysin Inhibition versus Enalapril in Heart Failure
Participants:
Originally adults with HFrEF (LVEF 40% or less), though this was changed to 35% or less by an amendment in the protocol.
Must have plasma BNP of at least 150 pg per mL (or NT-proBNP 600 pg/mL or greater)
This was lowered to BNP over 100 if they had been hospitalized for heart failure within last 12 months
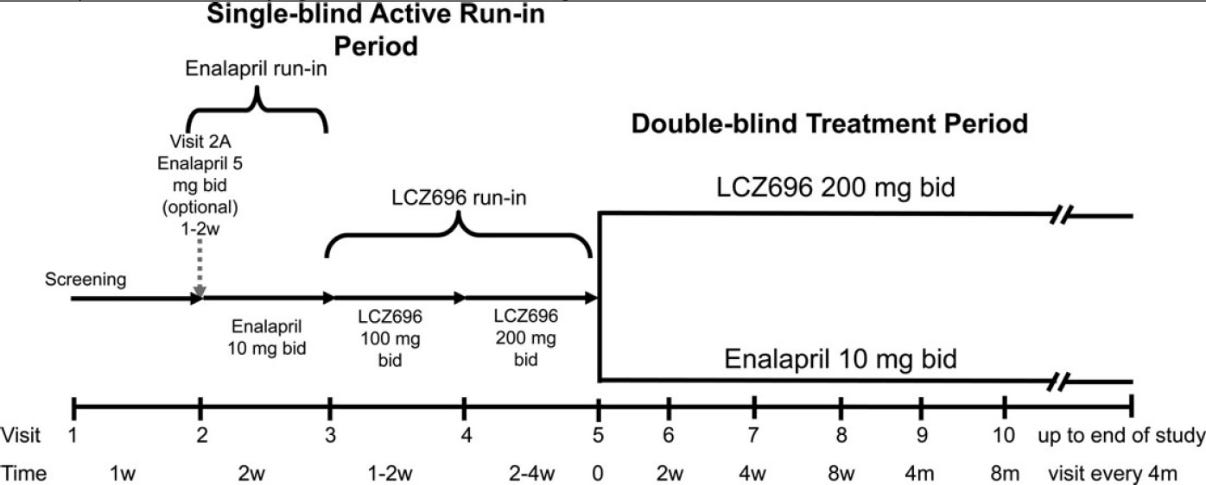
Design: There were three phases to this trial: a screening period, a single-blind run-in period during which all patients received Entresto, and finally a double-blind treatment in the two groups
Screening Period: At least 4 weeks on stable dose of beta blocker and ACEi/ARB equivalent to at least 10mg enalapril daily.
Single-blind run-in: All patients switched to Enalapril, 10mg BID, for two weeks. If no unacceptable side effects occurred, all patients were switched to Entresto for 4-6 weeks, initially 100mg BID, then 200mg BID. *The ARB component of Entresto 200mg is equivalent to 160mg valsartan.
Double-blind randomized treatment phase: Patients then randomized 1:1 to receive either enalapril 10mg twice daily or Entresto 200mg twice daily
Note: This is a bizarre design, and we will come back to it in more detail later, but let’s just move ahead for now.
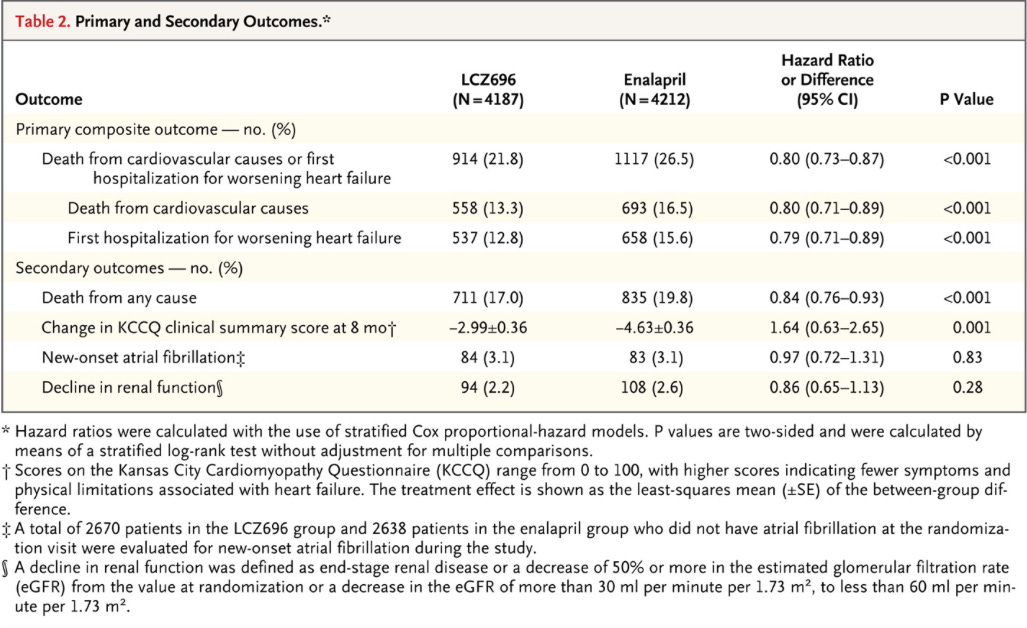
Primary Outcome: Composite of death from cardiovascular causes or a first hospitalization for heart failure
Secondary Outcomes: Time to death from any cause, change in clinical status via KCCQ questionnaire, time to new onset atrial fibrillation, and time to first occurrence of decline in renal function (ESRD, decrease of 50%+ in eGFR, or absolute decrease in eGFR of at least 30, to less than 60).
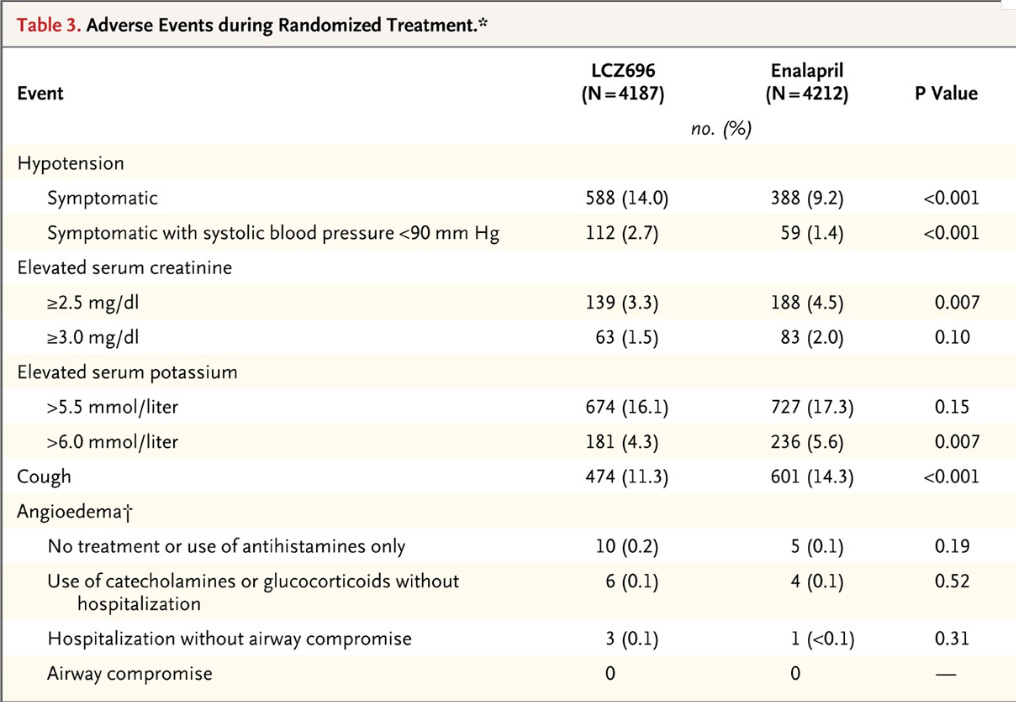
Results: Now you get to see some impressive results, I’ll let the figure and tables speak for themselves:
Now let’s see the adverse events:
Thoughts about this study:
We have seen some really promising outcome data. The all-cause mortality benefit is big. Here are a couple concerns with this study:
Asymmetry in dosing
Bizarre study design
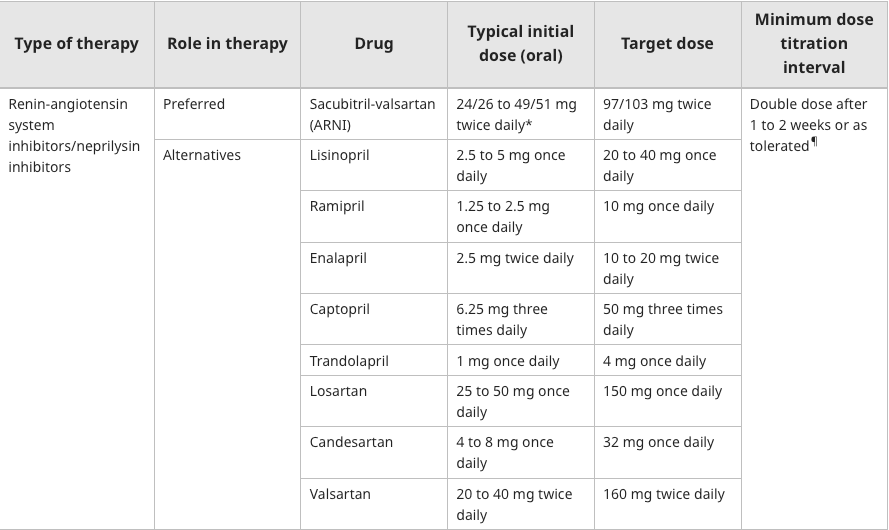
We can refer to the dosing chart for Entresto vs enalapril:
The target dose for Entresto is 97/103 mg BID, total daily dose of 400mg
The target dose for Enalapril is 10-20mg BID, total daily dose 20-40mg
In PARADIGM-HF: “At the last assessment, among patients taking the study medication, the mean (±SD) doses in the LCZ696 and enalapril groups were 375±71 mg and 18.9±3.4 mg, respectively.”
Note: “LCZ696” = Entresto.
In other words, Entresto patients were receiving ~94% of target dose on average
If we use 10mg BID for Enalapril, those patients were receiving 94.5% of target dose on average.
Problem: This is an issue, because Novartis decided to design the trial such that the max dose of Enalapril given was 10 mg BID even though patients could have been titrated up to double that dose. So Entresto gets to go head-to-head with a handicapped ACE inhibitor by design. Would the results have been the same if the enalapril group was titrated to a target of 20mg BID?
One rationale provided as an explanation is that in previous trials of enalapril, 10mg BID is often the target, rather than 20mg. This is a weak excuse in my mind, especially when you consider the study design. Every patient was able to tolerate that dose of Enalapril for the two week run-in, so it stands to reason that a good number of these highly selected patients could have tolerated higher doses. This brings us back to the study design.
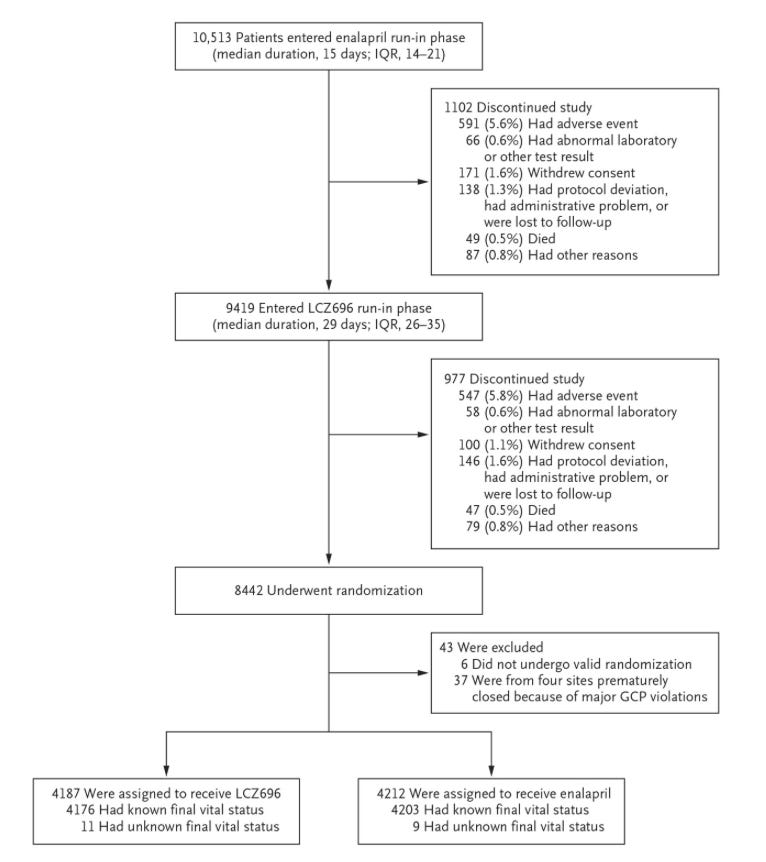
It helps to take a look at the phases, with the flowchart of patients below:
So you lose 20% of the population prior to randomization. These trial designs are slick, and they have a way of diluting the meaning behind “intention-to-treat” analyses. If you already get to exclude all the people who can’t tolerate the side effects of your drug before you randomize, you basically get to approximate per protocol and pretend that isn’t what you’re doing.
This is all not to mention the fact that Entresto has not been compared head to head with Valsartan for HFrEF in a trial powered for clinical outcomes. Next we look at a study that did compare the two.
PARAGON-HF (NEJM, 2019)
Title: Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction
Intervention: sacubitril/valsartan vs. valsartan
Entresto had target dose 200mg BID
Valsartan target dose 160mg BID
Participants: Ages 50+, NYHA class II to IV heart failure, EF 45% or higher, elevated natriuretic peptides and structural heart disease
Study Design: They did a similar run-in period design, except they only used half the target dose for each arm during their respective run-ins.
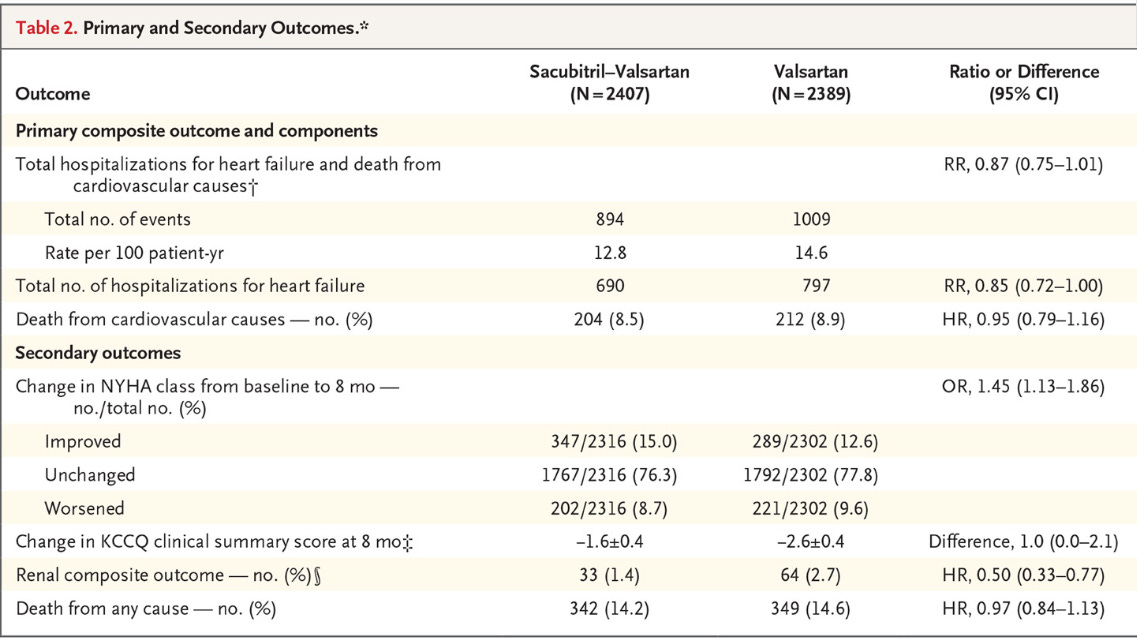
Primary Outcome: Composite of hospitalizations for heart failure and death from cardiovascular causes.
Results:
Safety:
Thoughts on PARAGON-HF:
This was a negative study, meaning they didn’t reach the primary end point. They came close, but this is nowhere near the benefit we saw in PARADIGM. Deaths from cardiovascular causes were unchanged, as well as all-cause mortality. There was directional benefit in heart failure hospitalizations, but overall it seems like the improvement was marginal at best.
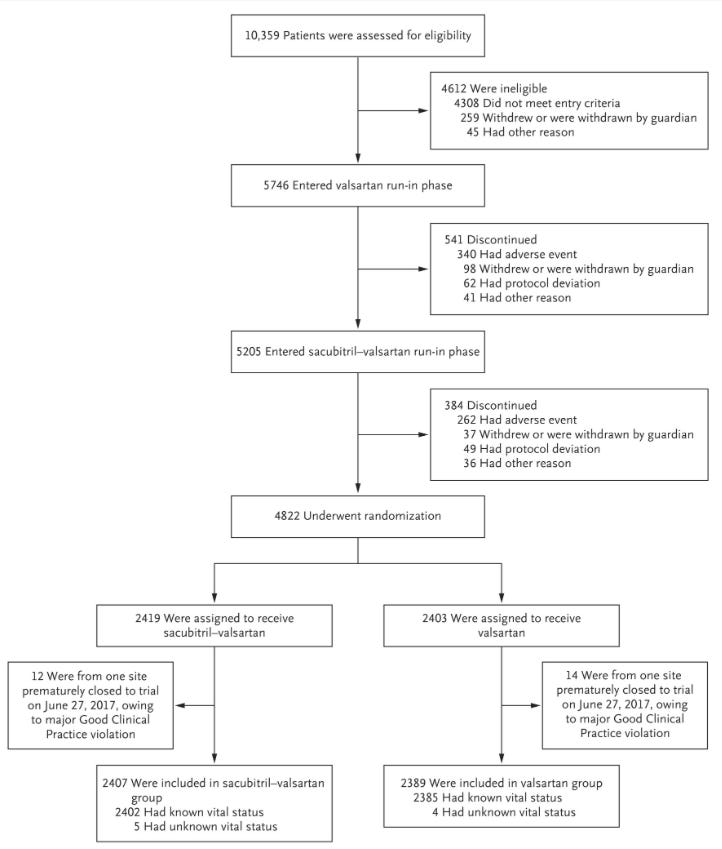
We once again see this terrible study design and huge dropout rates. This flowsheet shows how much drop-out there was prior to randomization:
The run-in phases lost 16% of the population that made it through screening. Even with this, the dropout rate following randomization was large:
“After randomization, 610 patients (25.3%) in the sacubitril–valsartan group and 638 (26.7%) in the valsartan group discontinued the trial drug for reasons other than death, and 370 patients (15.4%) in the sacubitril–valsartan group and 387 (16.2%) in the valsartan group discontinued the trial drug because of an adverse event.”
Final Thoughts:
There was another RCT, PARALLAX, that compared Entresto to standard treatment (ACEi, ARB) in patients with HFpEF. They measured NT-proBNP, 6 minute walk distance, NYHA class and quality of life measures. There was a reduction in BNP, no change in exercise, quality of life score or NYHA class. This was a negative trial, but worth mentioning due to its size.
My take on Entresto is that it never earned the “preferred” status it has in HFrEF.
Entresto was compared against valsartan in a phase II trial (PARAMOUNT). In that trial it had a positive effect on a biomarker. They had just 308 patients randomized, so they had no ability to assess hard outcomes.
PARADIGM-HF showed that 200mg BID sacubitril-valsartan was superior to 10mg BID enalapril in highly selected patients with HFrEF. There was no attempt made by Novartis to titrate the enalapril group as tolerated to the max dose.
PARAGON-HF showed that sacubitril-valsartan was not better than valsartan alone in patients with HFpEF. You could argue the directional hospitalization with heart failure numbers suggest benefit, but the deaths weren’t close.
Valsartan has been generic since 2012. Entresto was FDA approved in 2015. It is not clear to me that there is meaningful benefit of the sacubitril component of Entresto above and beyond valsartan alone. While Novartis may lose their patent later this year, my understanding is that they hope to delay any generic competition for another year at least. I know very little about patents and litigation around them, maybe one day that will be something to learn about.
In any case, it seems Novartis will end up having enjoyed roughly a decade of patent exclusivity. Novartis made $4.6 billion in sales of Entresto in 2022.