Nirsevimab is a monoclonal antibody that is designed to be given to infants to protect against RSV infection and its consequences. The FDA held an advisory committee meeting in June of this year and there was unanimous approval with respect to using this product as RSV prophylaxis for all infants during their first RSV season.
Today we will look at the major trial behind this decision, though there is another big one that will be mentioned.
MELODY was a double-blinded, randomized, placebo controlled trial. They followed a 2:1 randomization (nirsevimab:placebo). Saline was used as placebo.
Note: In March of 2022, the initial MELODY publication (linked above) was published in the NEJM. This presented data from 1,490 infants, 994 were randomized to nirsevimab, 496 to placebo. Basically they wanted to randomize 3,000 infants for the study, they paused enrollment during Covid, later resumed and ended up randomizing 3,012 infants. So the link above is the first cohort.
We will look at this data before looking at additional data later provided for the complete trial.
Participants: Healthy infants (<12 mo), born at least 35 wks 0 days, entering first RSV season. Full inclusion/exclusion is in the supplement. Infants could not have any current infection, previous RSV, LRTI, and could not meet criteria for palivizumab. Hence, healthy.
Intervention: Nirsevimab was given as a single intramuscular injection, 50mg if <5kg, 100mg if >5kg. Placebo consisted of single intramuscular injection of saline.
End Points:
Primary efficacy end point was medically attended RSV-associated lower respiratory tract infection (MA-RSV-LRTI) through 150 days after injection.
Secondary efficacy end point was RSV-associated hospitalization through 150 days after injection.
Results: Below is from intention to treat (ITT) analysis.
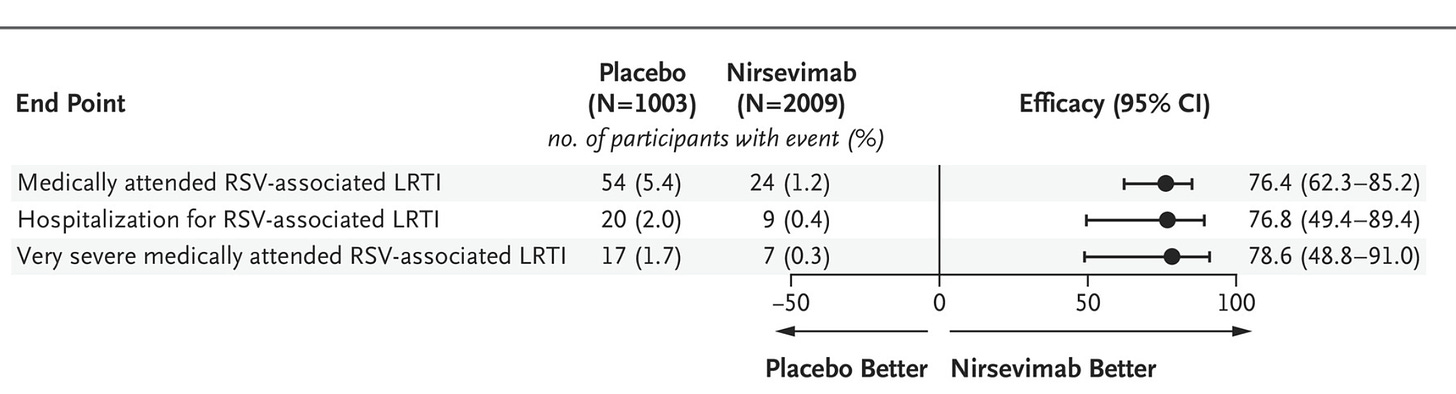
MA-RSV-LRTI: 12 events in the treated group, 25 in placebo. Note that with the 2:1 randomization this is a 4x difference. The primary outcome was observed in 1.2% of treated infants, 5% of infants that received the placebo.
Relative Risk Reduction was 74.5 (49.6 to 87.1).
RSV-Hospitalization: 6 events (0.6%) in the treated group, 8 events (1.6%) in the placebo group.
Relative Risk Reduction was 62.1 (−8.6 to 86.8)
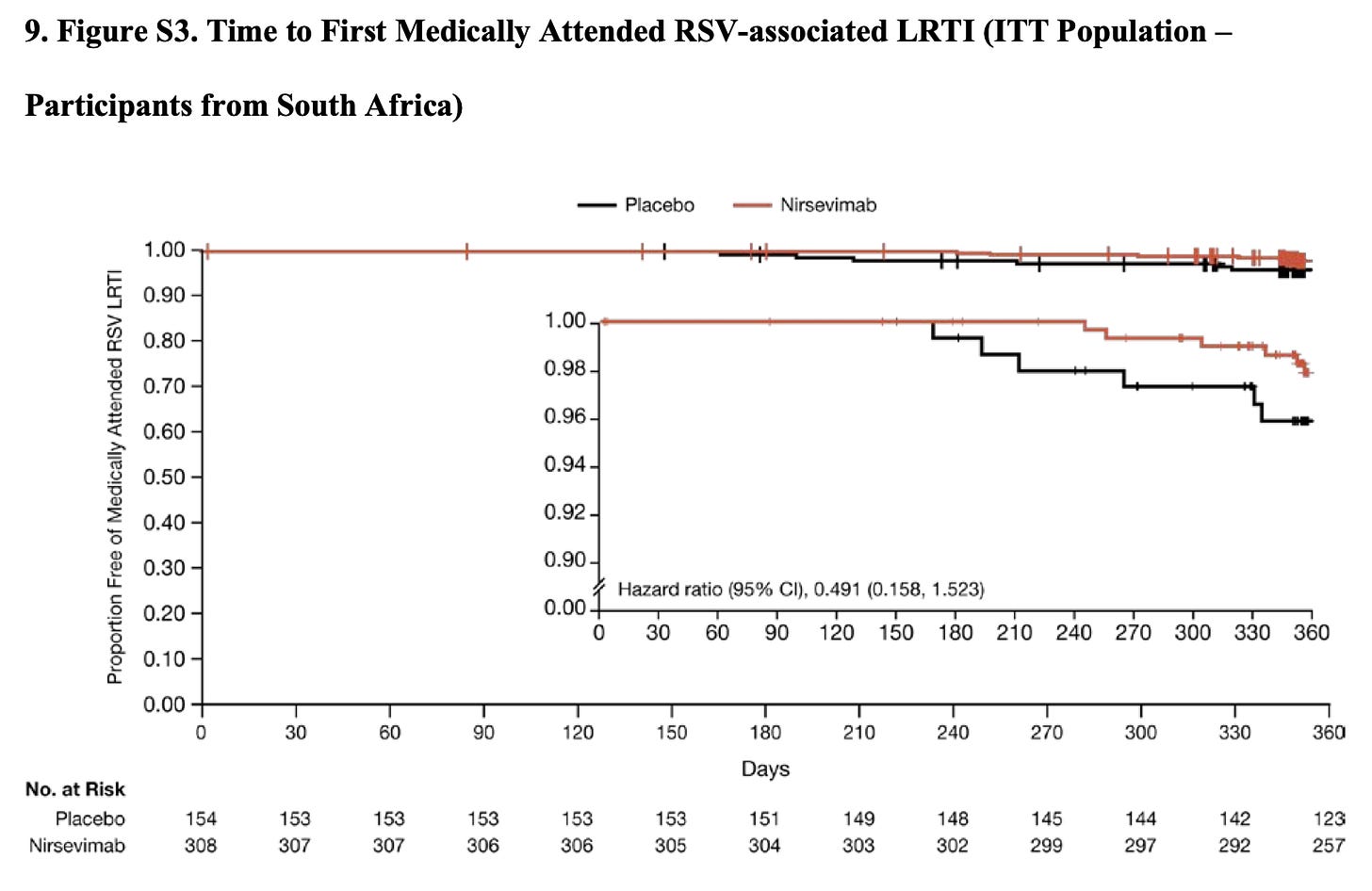
Side Discussion: They were running this trial in both northern and southern hemispheres, and southern hemisphere recruitment was entirely in South Africa. Of the nearly 1,500 infants enrolled, 462 were enrolled in South Africa. So roughly a third of this cohort. When Covid hit, South Africa’s response to the pandemic resulted in there being zero cases in the 150 days following intervention.
This probably dilutes the ITT efficacy data we saw above. While no events occurred in South Africa by day 150, infections beyond that point did suggest there is continued protection:
Safety: Serious adverse events occurred at similar rates, in 67 (6.8%) of nirsevimab recipients and 36 (7.3%) of placebo recipients. There was no anaphylaxis, a single adverse event of special interest was reported. One infant that received nirsevimab had a generalized macular rash that began 6 days after administration. There were no systemic features, no treatment was required, and the rash resolved after 20 days.
There were 3 deaths in the nirsevimab group, none in placebo. Below is the description provided in the supplement. I don’t think you can draw any conclusions from that number of events.
Initial Thoughts for first cohort: The efficacy data here is definitely promising. MA-LRTI data is strong, hospitalizations data also promising but far fewer events. The pandemic and what happened with South Africa likely diluted the efficacy data, but there was some suggestion of efficacy beyond 150 days as well. For anyone concerned about the asymmetry in deaths, it is good that we will have another cohort to look at shortly.
Second Cohort: In April 2023 we got updated data with the full 3,012 participant data set.
Efficacy Update: The efficacy data is impressive.
Here we can start with RSV-associated data:
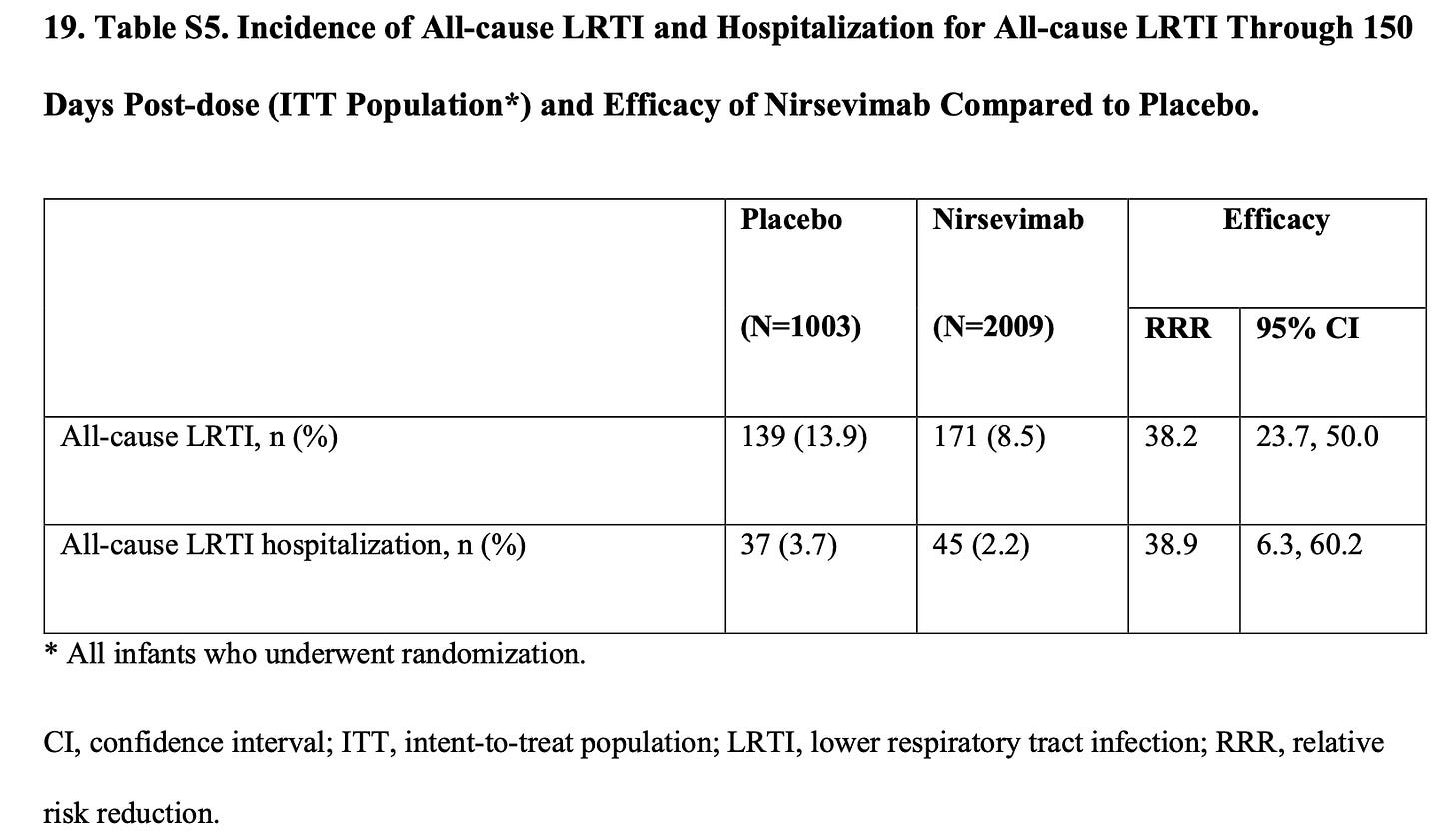
And then we can look at all-cause data:
Safety Update: In the update they listed four hypersensitivity events, limited to cutaneous findings. It sounds like there were three more episodes of rash, still no anaphylaxis. For those concerned about the initial 3 deaths, the full data set had one additional death, which was unambiguously unrelated to the intervention. The supplement includes that the death was due to involvement in an automobile accident.
I think overall the safety data is pretty solid. Of course it would be nice to have a larger data set, and there is additional safety data when you include other trials that the FDA looked at.
Another relevant trial: Though I won’t discuss it in this post, nirsevimab was studied in healthy infants born from gestational age 29 wks 0 days to 34 wks 6 days. Basically identical trial design, similar results. Here’s the link.
Some Thoughts: Now the tricky thing of course is what to do with this data. There are some unanswered questions. If the maternal RSV vaccine is approved, we have no data on this product being used in infants of Abrysvo recipient mothers. I also think the whole second season data was limited.
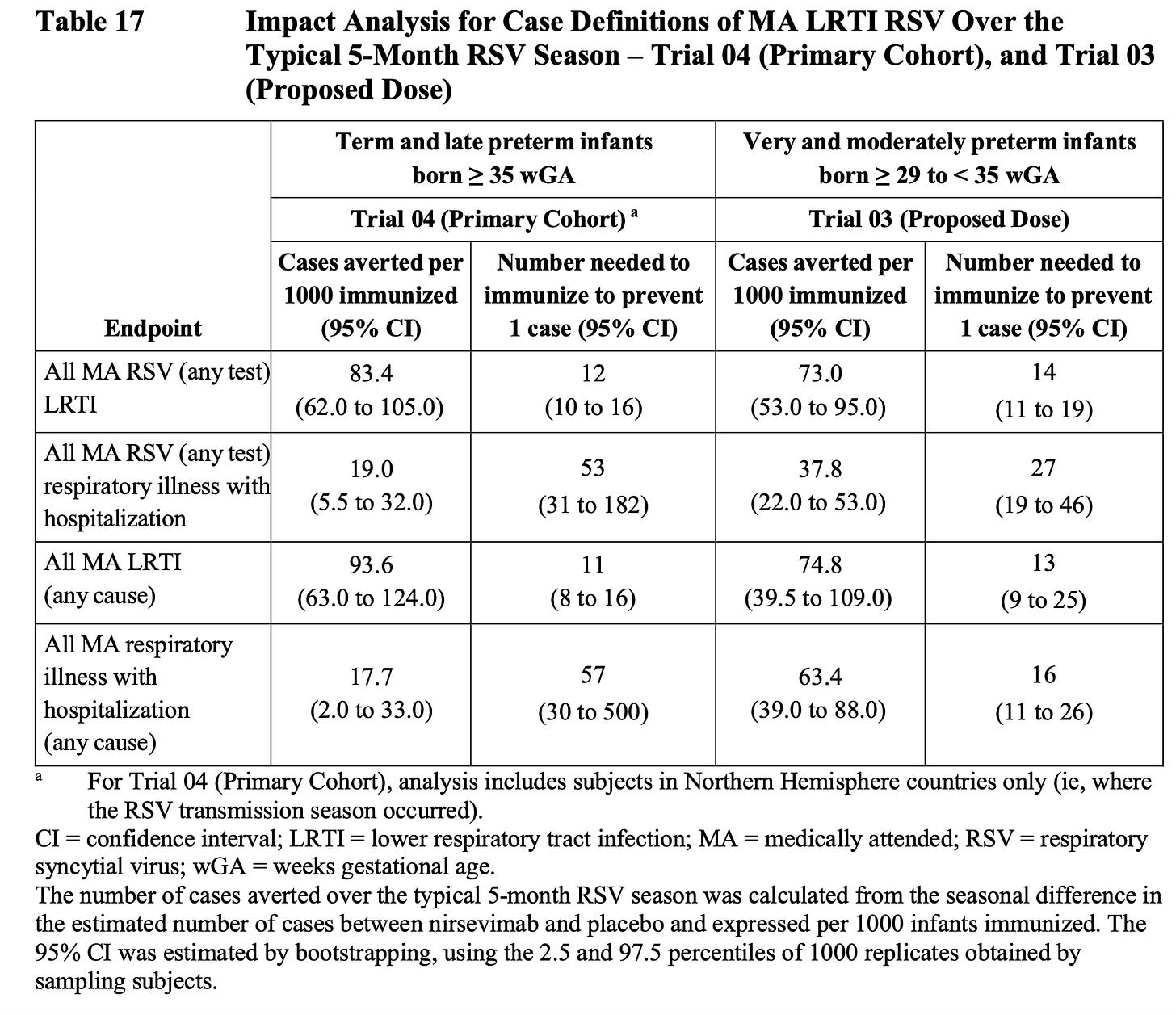
Below is a snapshot from the FDA briefing doc, which is a great resource for a deep dive. I just assume that these NNT numbers based on clinical trials are overly optimistic by at least half.
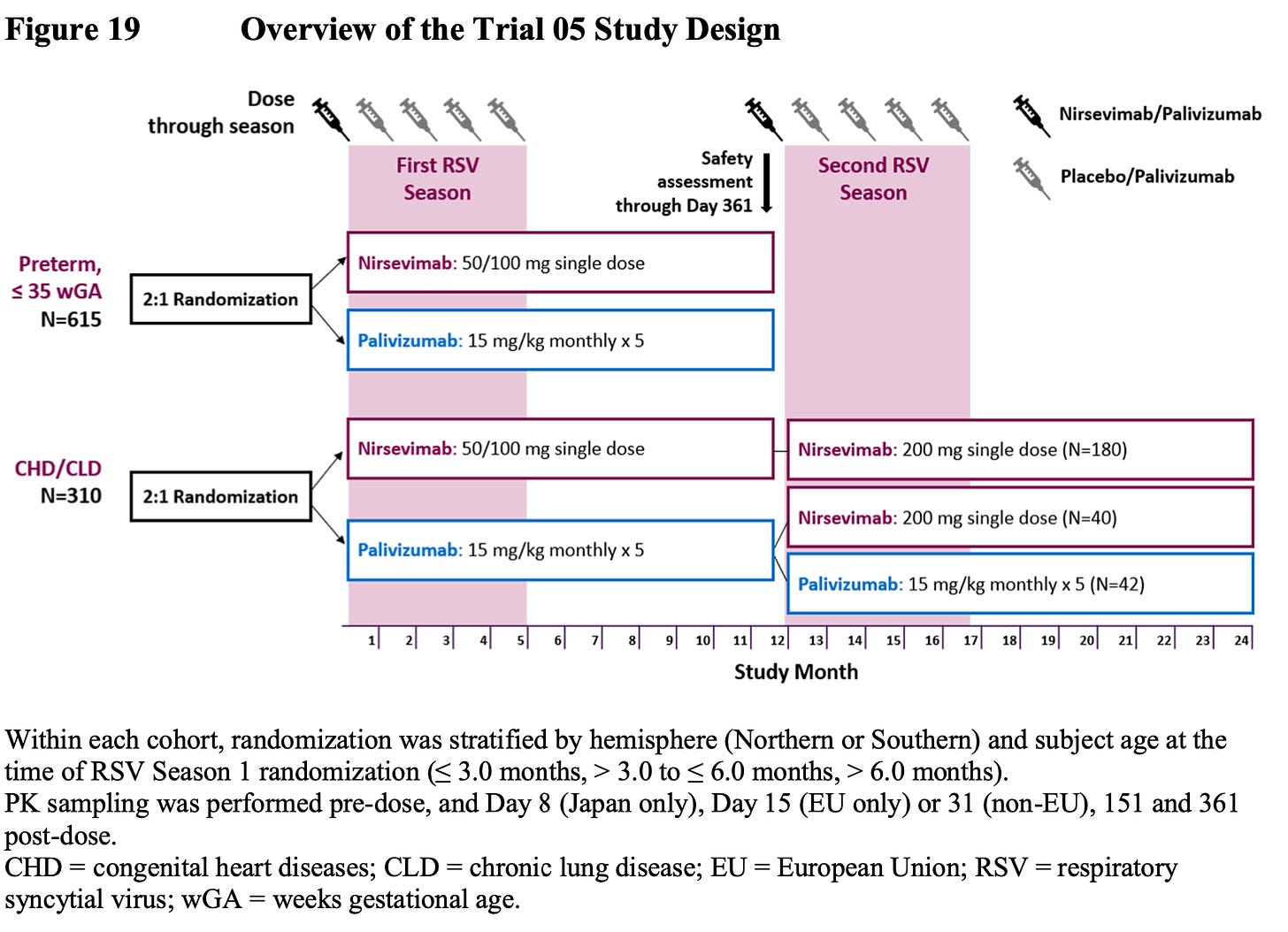
For higher risk infants, they did do a head to head trial of nirsevimab vs. palivizumab. The trouble is they only assessed relative safety, not efficacy. They extrapolated efficacy data by basically saying, “It worked in healthy preterm and term infants, so it should work in unhealthy ones.” I’m not really a fan of that assumption, even if I might assume it’s correct. Here is the design of that trial:
Final Thoughts: I’m left feeling like this could be a great product, but I’m personally made uneasy by the sample size. It's a huge ask to start recommending this for every single infant. I would prefer being able to risk stratify further. At the end of the day I personally have a high threshold of proof for any intervention. I think these studies have demonstrated impressive efficacy. I’m left wanting another 10X of the sample size with respect to safety. I will say I’m far less skeptical of this product than maternal administration of Abrysvo.
I just don’t think a sample size of ~2,500 drug recipients for total safety data will ever satisfy me for this kind of approval. My threshold for safety data in healthy infants is extremely high. In my mind I would need a few things to feel great about this drug:
I would need to see 10,000 nirsevimab recipients with excellent safety profile, plus all-cause mortality improvement or huge reduction in hospitalizations.
I would like complete follow up for probably two seasons, you could even do another randomization in the treated arm to dose again in season two.
I would also not extrapolate efficacy data to less healthy infants.
I guess that’s medicine though. We have imperfect information, people have different levels of risk tolerance with respect to infections, medications, etc. FDA approval seems reasonable, and will probably happen in the coming months. Uptake will be interesting, especially depending what happens with Abrysvo. I’ll probably be griping about safety data for the rest of my life, maybe if I’m merely uncomfortable that means it’s a fairly safe medication.
Edit: Some additional data and thoughts on recommendation
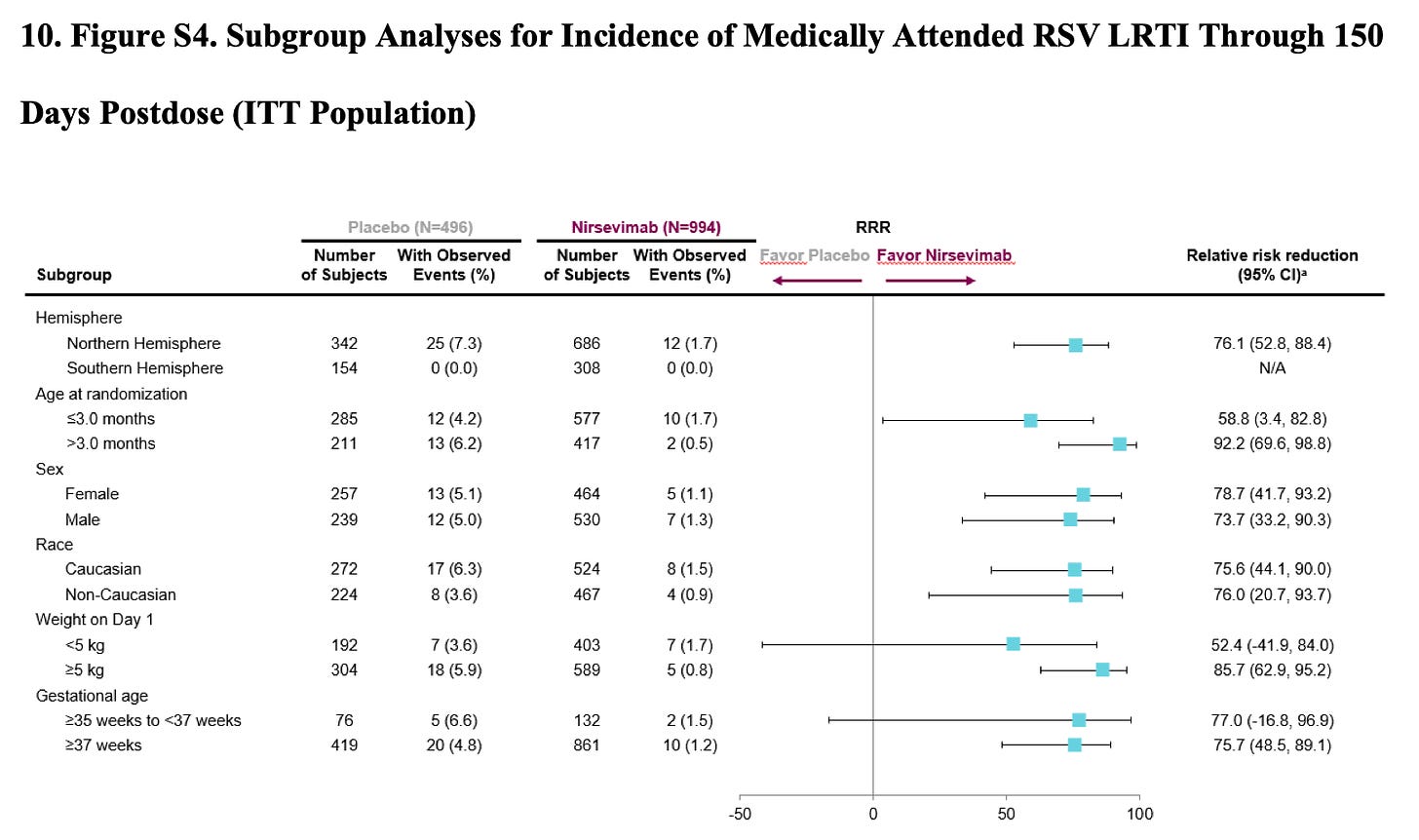
I originally omitted this but realize it would be better to include. One big problem with the way MELODY is designed is that it lumps in babies born from 35-37 wks with term infants. Preterm birth is a risk factor for severe RSV, and including this higher risk could inflate the disease burden in this cohort, thereby making the treatment appear more beneficial. If a huge chunk of the benefit is clustered among those infants born from 35-37 weeks, this weakens the argument that any recommendation might apply to the group as a whole.
There is some data on this. Below is the outcome data in the first ~1,500 infants in MELODY stratified by gestational age.
This data is at least somewhat reassuring, as it shows that ~80% of the cases were seen at 37 wks plus. But this is only half of the MELODY data, and it is for the softer end point of MA-LRTI, not hospitalizations. I could not find the same stratification for the complete cohort.
I do think the data that impressed me was the ~1.5% absolute risk reduction in hospitalizations. But taking a risk of hospitalization from something like 2% to 0.5% is not enough to justify giving this product to every infant moving forward. If you were doing that same thing except with mortality then I would be on board. Very few healthy infants, especially those born at term, will die from a bout with RSV.
When I say I think FDA approval is reasonable, I should be clear that I don’t think all healthy infants should necessarily receive this product. I don’t think there is enough safety data, both in absolute numbers and length of analysis, to be comfortable saying it makes sense to give this to all infants. Something like a universal recommendation for a product targeting a common childhood illness should have more data behind it.
There is a more fundamental critique of the enterprise that can be made. RSV is said to cause something like 100-300 deaths of children <5 years old each year. That is out of something on the order of 20 million children. Every one of those deaths is a tragedy, but it’s clear that if nirsevimab can reduce mortality, the number needed to treat to save a life would be massive. And we’re talking about healthy children, how many of those deaths each year are in high risk children, for whom the efficacy of nirsevimab has been “extrapolated”.
I might be veering towards rambling so I’m going to end this addendum.
Happy Friday




I had a preemie born at 33 weeks in October and nothing was offered. That particular year we did have some sort of LRTI at around 37 weeks? (He'd been home a few weeks when he got sick.) We never saw a doctor and managed it at home without complication. Given what we know about the risk of RSV to the frail elderly when the immune system starts to wane in old age, I'd question if there is lifetime risk to this intervention and how the development of natural immunity to an ubiquitous virus is impacted by the mass administration of something like this.
I don't feel comfortable with mass administration of something like this without a whole lot more data points - what happens immediately after is just one part of the picture. What happens by the time they're 5, 6, 10, 30 years old? It seems lately that medicine has a habit of short term gain focus, without concern for long term risk. And yet we all hope to live into the long term, so it would seem to me that this is a really risky approach.
Thank you for this analysis. I have several concerns that you didn't mention.
35-37 week EGA infants are not equivalent to term infants in terms of risk of severe RSV illness. I don't find cases or severe cases broken out by gestational age, including in the supplement. Maybe I missed that?
Although the relative risk reduction numbers are large, the actual numbers for risk reduction are small. The primary endpoint of medically attended LRTI is defined as a baby who comes into an outpatient setting coughing, with rales or wheezing. The vast majority of those babies recover uneventfully after several days of supportive care at home. Reducing the number of babies who experience that from a single digit percent to a lower single digit percent may ultimately be considered valuable enough, but doesn't seem sufficient to warrant a universal recommendation for a new therapy. Very severe medically attended LRTI is defined as any child who received supplemental O2 or IV fluids in hospital, basically just about any child admitted for bronchiolitis. (Severe, maybe, but the "very" seems like a sales pitch:) Again a decrease in this from 1.7% to 0.3% doesn't seem to warrant a universal, new, likely expensive drug. More data on length of stay in the severe cases, ICU admission and gestational age would have been helpful. Based on long experience as a pediatrician, I would guess that most stays were 2-4 days, and the longer stays with ICU time were predominantly late preterm infants. Again, using data including preterm infants to justify recommendation of this treatment for term infants seems pretty tenuous.
Like the previous commenter, I would also have concerns about the population impact of kicking the first RSV illness down the road a year in healthy children, though it will just happen the next year not 5 or more years later. This is obviously good for very high risk children, but may not be for healthy infants. Maybe it's better to get your first RSV when you still have maternal antibodies around? We don't know yet.
Cynically, I'm braced for a big push for more RSV testing and maybe a declaration of public health emergency to drive quick uptake of this when it should be assessed more carefully. More fear and disruption for parents, as well as a big economic investment for small gains may be the major adverse effects.