Lipid-lowering therapies (eg, statins) reduce the risk of cardiovascular events and save lives. PCSK9 inhibitors represent an exciting class of drugs with potent lipid-lowering capability. In recent years two of these drugs have been approved in the United States. We will cover some of the background of these drugs, the basics of FOURIER, and the findings and concerns brought up by a recent analysis.
Background
Drug Name: Evolocumab (brand name Repatha)
Drug Class: Evolocumab is a human monoclonal antibody that binds to proprotein convertase subtilisin kexin type 9 (PCSK9).
Relevant Physiology + Mechanism of Action: PCSK9 is a protein in the liver that binds to low-density lipoprotein receptors (LDLR), leading to their degradation. LDLR is the primary receptor that clears circulating LDL. By inhibiting PCSK9, Evolocumab increases the number of LDLRs available to clear LDL from the blood, lowering LDL-C levels.
*Note: I will use “PCSK9i”, the “i” denotes “inhibitor(s)” for ease.
Repatha was not the first PCSK9i to receive FDA approval. Alirocumab (brand name Praluent) was originally a month ahead, as we will see.
Drug Developers: Amgen makes Evolocumab (Repatha). Sanofi and Regeneron make Alirocumab (Praluent).
Timeline of PCSK9i approvals:
July 24, 2015: FDA approves Alirocumab (brand name Praluent) in certain patients for its ability to further reduce LDL-C.
August 27, 2015: FDA approves Evolocumab for the same reason.
December 1, 2017: Following the FOURIER trial, the FDA approves Evolocumab to prevent heart attack and stroke in adults with established cardiovascular disease.
April 26, 2019: Following the ODYSSEY trial, the FDA approves Alirocumab to prevent heart attack, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular disease.
FOURIER Trial
Basics:
This was a randomized, double-blind, placebo-controlled trial with 27,564 patients randomized to either Evolocumab or placebo.
Eligible patients were 40-85 years old, had established atherosclerotic cardiovascular disease (previous MI, non-hemorrhagic stroke, or symptomatic peripheral artery disease) plus additional cardiovascular risk factor(s).
Patients also had to have a fasting LDL-C of 70mg/dL per deciliter or higher OR a non-HDL-C of 100mg/dL or higher while taking an optimized regimen of lipid-lowering therapy.
Primary End Point: Major cardiovascular events, defined as the composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization.
Key Secondary End Point: Composite of cardiovascular death, myocardial infarction, or stroke.
The median duration of follow-up was 26 months.
Results:
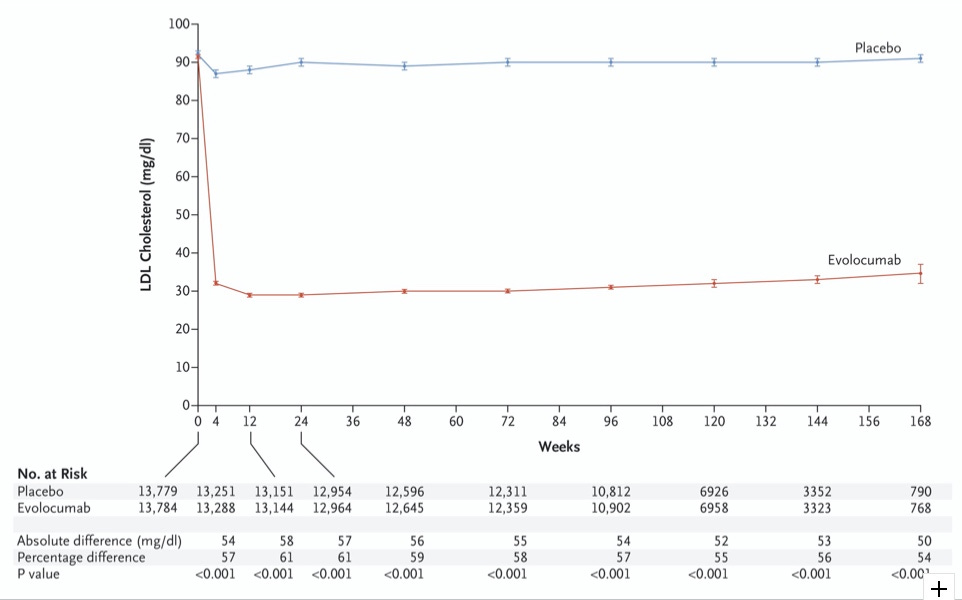
Evolocumab eviscerates LDL-C (Figure 1):
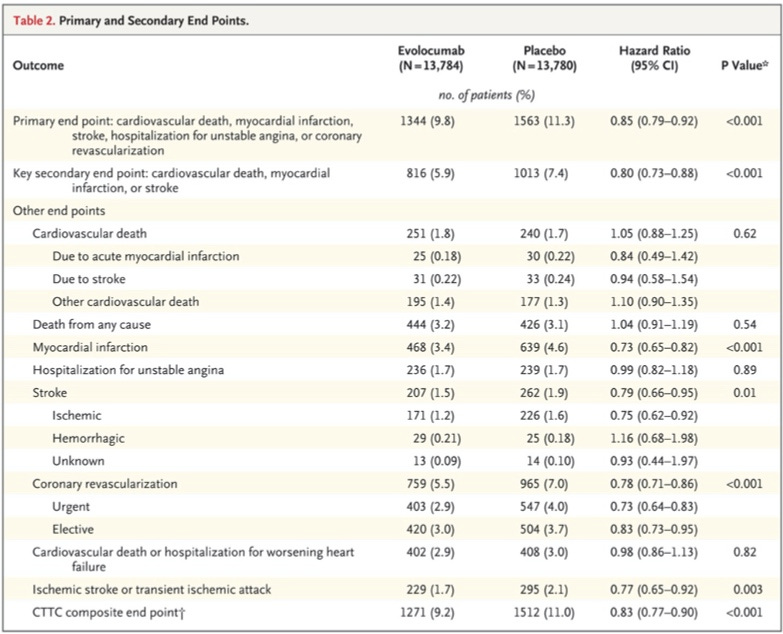
“The primary end point occurred in 1344 patients (9.8%) in the evolocumab group and in 1563 patients (11.3%) in the placebo group (hazard ratio, 0.85; 95% CI, 0.79 to 0.92; P<0.001)”
“The key secondary end point occurred in 816 patients (5.9%) in the evolocumab group and in 1013 patients (7.4%) in the placebo group (hazard ratio, 0.80; 95% CI, 0.73 to 0.88; P<0.001)”
Here is the full results table:
My Thoughts and Interpretation:
Lipids: This drug is really good at reducing LDL-C. I also appreciate that they reported the apoB reduction, which was cut in half. I’m pretty confident that is a great thing, all else being equal.
Primary and secondary end points: Similarly impressive stuff. Even more so when you consider these reductions were accomplished with a median follow-up of just over two years!
Other end points: First thing to point out is the study was powered for the key secondary end point, the authors “…estimated that 1630 such end-point events were required to provide 90% power to detect a 15% relative risk reduction with Evolocumab as compared with placebo.”
That being said, there was no effect on cardiovascular death or death from any cause. Both of these outcomes were slightly more likely to occur in the treatment group, but this was not statistically significant and they weren’t powered to analyze this difference.
The lion’s share of the benefit based on the table seems to come from a reduction in non-fatal myocardial infarction.
Overall, I would say this data is quite promising and I wish they had continued the studied for the four years of median follow-up originally planned. The all-cause mortality at least would have likely reached the event threshold by that time.
The Plot Thickens
Here we will introduce a paper I read this morning. This was a reanalysis of the FOURIER trial outcomes based on regulatory data, published in BMJ Open on December 30th, 2022. Do read the whole thing, linked above, but we will go through an overview.
Rationale: In FOURIER, both cardiovascular and all-cause deaths were numerically increased in the Evolocumab group, as we have seen. Given the reported statistically significant reduction in myocardial infarction and stroke (this is what the FDA went on to approve its use for), one would not expect the mortality data to be directionally pointing the wrong way.
Methods: The authors analyzed the Clinical Study Report (CSR), “..a technical document prepared by the manufacturer and submitted to regulators as part of the approval package for drug evaluation. It contains information about the trial’s protocol, amendments, inclusion and exclusion criteria, outcome definitions and measurement, efficacy and safety results and statistical analysis plan. The main FOURIER CSR is over 25 000 pages.”
Access to the data: The group was able to access the CSR via Health Canada, which made the data public in 2020 in accordance with a law designed to promote transparency and accountability regarding drug approvals. When they requested the data from the FDA they were informed it would take 3-4 years to address the petition and another 2-3 years to release the information.
Results: Due to limited data available in the CSR, they were only able to analyze deaths, and had no access to the data underlying non-fatal myocardial infarctions or strokes. I will directly quote major results from the paper, here is the link again.
“When comparing the CSR information with the 2017 NEJM publication, we found 11 more deaths from myocardial infarction in the evolocumab group (36 vs 25) and 3 less deaths in the placebo group (27 vs 30, respectively). One of the most frequent causes of death we identified by readjudication was ‘cardiac failure’. The 2017 NEJM article did not report this outcome. Readjudication indicated that deaths attributable to cardiac failure in the evolocumab group were almost double those in the placebo group (31 vs 16, respectively).”
“The 2017 NEJM publication reported that cardiovascular mortality was non-significantly increased in the evolocumab group (251) versus placebo (240), HR=1.05 (95% CI, 0.88 to 1.25), p=0.62. After readjudication, we found a greater (although still non-significant) relative increase in cardiovascular mortality in the evolocumab treatment arm: RR=1.20 (95% CI 0.95–1.51), p=0.13.”
My Thoughts: I don’t know what the final word will be on this topic or this drug. I hope there will be a response from the authors, the FDA, and additional analysis. My deeper concern is with the status quo of oversight of trials that lead to approval of drugs in general. The FDA apparently would have taken 5-7 years to provide the CSR, and that says nothing about the underlying CFR (Case Report Forms), which represent the actual primary data for studies.
How can physicians feel comfortable prescribing these medications if we don’t have robust checks on Pharma companies and access to their underlying data? I hope to do a post at some point on the basics of how drug approval trials are run, who actually gets access to the data, etc. This stuff is well known by people steeped in it but is fairly shocking to learn.
P.S. I am not arguing PCSK9i don’t work or are killing people. I think that should be clear, but I do think these authors brought up legitimate concerns regarding the FOURIER trial analysis that will have to be addressed. I remain bullish on PCSK9i overall, Praluent’s data from 2018 doesn’t show the same directional issue with mortality.



Have you read the book “The Great Cholesterol Myth: Why Lowering Your Cholesterol Won't Prevent Heart Disease-and the Statin-Free Plan That Will”? Their analysis of current cholesterol/statin treatments as well as the studies they’re based on made me question that methodology. It was actually the precursor for my eventual mistrust of big pharma with the c19 shots.