Brief Background
Colchicum autumnale, pictured below, is a beautiful and deadly plant. It’s toxicity is due to the colchicine content within the plant. The earliest known documentation of its use as a medicine goes back to 1500 BC, when it was inscribed on Egyptian papyrus. At the time it was used for joint pain, and to this day it is used to treat gout, an inflammatory arthritic condition.
How does colchicine work?
We can start with the brief description that appears in the paper we will focus on, “…colchicine has broad cellular effects that include inhibition of tubulin polymerization and alteration of leukocyte responsiveness.”
There has been recent interest in the use of this ancient medication to prevent major adverse cardiac events. It is thought that inflammation contributes to things like heart attacks, and blocking inflammatory pathways may benefit patients.
The “alteration of leukocyte responsiveness” mentioned above is more specifically inhibition of neutrophils and inflammatory pathways they mediate. From what I have read, this is the most commonly suggested mechanism that may confer a cardioprotective effect.
Back in June of this year (2023), the FDA approved Lodoco (colchicine), “…to reduce the risk of myocardial infarction (MI), stroke, coronary revascularization, and cardiovascular death in adult patients with established atherosclerotic disease or with multiple risk factors for cardiovascular disease.”
The FDA cites the LoDoCo2 trial as justification for this approval. Consequently, we will focus on this trial.
Note: LoDoCo2, think low dose colchicine. Published 2020, NEJM.
“Colchicine in Patients with Chronic Coronary Disease”
Inclusion Criteria: This was a secondary prevention study. Patients had to have evidence of established cardiovascular disease and be clinically stable for at least 6 months before enrolling.
Exclusion Criteria: “Patients were not eligible if they had moderate-to-severe renal impairment, severe heart failure, severe valvular heart disease, or known side effects from colchicine.”
The study was run in two countries. Australia and the Netherlands.
Run-in phase: 6,528 patients entered an open label run in phase where they gave the study drug at full trial dose, 0.5 mg daily for one month. 15.4% of patients dropped out during the run-in, the most common reason of which was gastrointestinal upset.
5,522 patients underwent randomization, 1:1 to either continue 0.5mg daily colchicine or switch to matching placebo. At this point the study was double-blinded.
Note: I don’t love this study design for a few reasons.
Screening out patients who can’t tolerate the drug in the run-in makes the medication appear more tolerable.
Given the high rate of GI discomfort, this potentially weakens blinding in the study. Assuming the placebo doesn’t cause GI upset, patients with discernible symptoms that were able to tolerate the run-in might be unblinded when switched to placebo.
This adds the variable of discontinuation of colchicine.
End points:
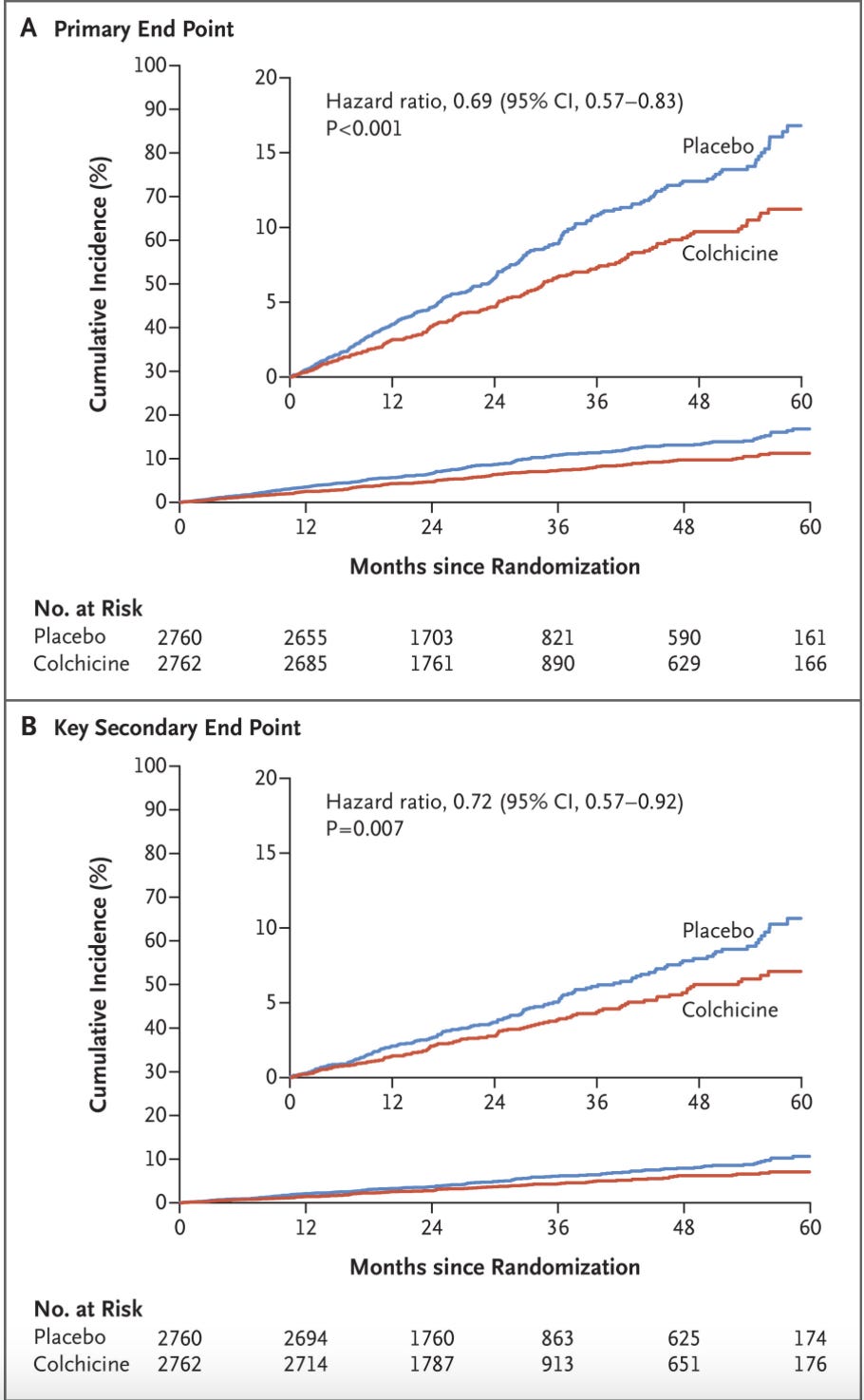
Primary Endpoint: “…a composite of cardiovascular death, spontaneous (nonprocedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization”
Key Secondary Endpoint: “composite end-point event of cardiovascular death, spontaneous myocardial infarction, or ischemic stroke”
Results:
My thoughts: The primary end point result looks great. There are a couple things that stand out though. First and foremost is the non-significant but directional increase in all-cause mortality. 73 deaths in the treatment arm, 60 in placebo. We can also see that the improvement in the primary outcome was driven by a reduction in myocardial infarction and ischemia-driven coronary revascularization.
When focusing on non-cardiovascular death, the increased rate in the treatment group narrowly avoided statistical significance:
There was also an interesting geographical difference between Australia and the Netherlands. From the supplement (look at the bottom of the figure):
Roughly two-thirds of patients were randomized in the Netherlands, where it looks like the primary end-point was a wash. In Australia, the benefit from colchicine was profound. This raises a couple of questions in my mind. My first thought was to compare event rates in treatment vs. placebo in each country.
Netherlands + Placebo: 117/1807 = 0.065, or 6.5% of patients experienced an event
Netherlands + Colchicine: 109/1811 = 0.060, or 6% of patients experienced an event
Australia + Placebo: 147/953 = 0.154, or 15.4% of patients experienced an event
Australia + Colchicine: 78/951 = 0.082, or 8.2% of patients experienced an event
It appears patients in Australia were at far higher risk. My first thought was to compare baseline characteristics, which were provided in the supplement.
There are a handful of differences we can see here. In my mind the one that stood out most is kidney function. In the Netherlands, 7.9% of patients had CKD stage 3a (eGFR 45-59), versus only 1.1% in Australia. Colchicine is cleared by the kidney, but I’m not sure whether a mild reduction in function would have a significant impact on drug clearance, and this doesn’t explain the heightened risk in the Australian cohort.
Drug-drug interactions: Colchicine is metabolized by CYP3A4 in the liver. Some calcium channel blockers can inhibit this enzyme, leading to increased levels of colchicine, but the difference in usage at baseline seems insignificant.
Time at risk: Finally a lightbulb went on and I remembered the following from reading the methods:
Patient recruitment began at 13 centers in Australia in August of 2014.
Recruitment was expanded to 30 centers in the Netherlands in October 2016.
Enrollment was completed by December 2018.
The date of last follow-up was February 2020.
I think this suggests an explanation for the regional difference that would be favorable to colchicine’s efficacy. The authors did not provide data on the duration of treatment/follow-up stratified by region. We do know that median follow-up overall was 29 months (IQR, 20.7-45.7) in the treatment arm, 28.1 months (IQR, 20.3-43.5) in placebo.
So I went to the correspondence on the paper, reasoning that someone else would have pointed this geographic asymmetry out, and the authors would have provided data to explain it.
Note: When papers are published, people can send in commentary, often in the form of a “Letter to the Editor”, and if selected by the journal then the authors have a chance to respond. When I refer to “correspondence on the paper”, this is what I mean. If you have the study open on NEJM it is at the bottom.
I was correct in assuming that multiple letters included the observation about Australia vs. Netherlands, but the authors response was mostly limited to urging caution about subgroup interpretation, which is reasonable.
Correspondence
So I reached out to one of the authors on the study and suggested my interpretation of the asymmetry as possibly secondary to delay in enrollment in the Netherlands relative to Australia. Per his response, this hypothesis was correct and the duration of follow-up mediated the geographic difference. He shared convincing data to that effect, which he says is part of a paper that is currently under review. I will keep an eye out for that paper and maybe discuss this topic again briefly when it comes out.
Final Thoughts
Here is an excellent paper that reviews the major RCTs in this space. “COLCOT” studied colchicine vs. placebo initiated within 30 days of a myocardial infection. Primary outcome was positive, all-cause mortality was basically identical. “COPS” (Colchicine in pts with acute coronary syndrome) was a relatively small study (n=795) which had 8 deaths in the colchicine arm, only 1 in placebo. Both of those studies assessed colchicine use in the more acute setting, as opposed to stable CAD in LoDoCo2.
I think this paper is encouraging with respect to the use of Lodoco for cardioprotection. I still don’t know what to make of the all-cause mortality data. I asked the author for his thoughts on this issue and will share any forthcoming explanation in the comments. I’m left scratching my head a bit on this one, let me know if you have any thoughts.








Is it possible that all cause mortality is the hard end point, whereas there can be error and subjectivity in the primary end point? Death is fairly straightforward to diagnose but I understand that determining the primary endpoints can be much more prone to error.
Thanks for your analysis of this article.