It’s my pleasure to introduce today’s guest post by Dave Allely. Although Mr. Allely is a medical student— his analysis of the RSV vaccine for pregnant women is superior to the FDAs. He notes a bizarre moment in the recent VRBPAC meeting where Peter Marks asked Pfizer to make a closing statement— seemingly in an effort to get the vote he wanted. I wonder why Peter Marks has repeatedly pushed Pfizer products onto the US market despite uncertainty. I worry that he is acting more as a representative of Pfizer than an impartial regulator.
Vinay Prasad MD MPH
On Thursday, May 18th, the VRBPAC (Vaccines and Related Biological Products Advisory Committee) convened for their 181st meeting. On the agenda was Pfizer’s maternal RSV vaccine, Abrysvo. Pfizer is seeking FDA approval for Abrysvo, and the VRBPAC meeting is an important hurdle for pharmaceutical companies seeking licensure.
The committee was tasked with voting on two questions, which I will include in full here:
Are the available data adequate to support the effectiveness of immunization with ABRYSVO during the second or third trimester of pregnancy (24-36 weeks gestational age) to prevent RSV lower respiratory tract disease [LRTD] and severe RSV LRTD in infants, from birth through 6 months of age?
Are the available data adequate to support the safety of immunization with ABRYSVO during the second or third trimester of pregnancy (24-36 weeks gestational age) to prevent RSV LRTD and severe RSV LRTD in infants, from birth through 6 months of age?
For the first question (effectiveness), the committee voted unanimously (14/14) in the affirmative. For the second question (safety), four members voted “no”, ten members voted “yes”.
The available data consisted almost exclusively of the data from the MATISSE trial. Readers of Sensible Medicine or Knave Notes may recall my analysis of this trial from just over a month ago. I will not fully recapitulate that post here, so it may be helpful to provide context and some definitions. My concerns were broken down with respect to efficacy, effectiveness, and safety. Here I will expand on these concerns and share why I disagree with the committee.
Note: The FDA uses the colloquial, rather than technical, definition for “effectiveness” in the first question. If you are used to the technical terms, just think “efficacy”.
Before we talk about safety and efficacy, I’d like to share a moment from the meeting that left me dispirited. At the end of the meeting there are two hours set aside for the committee to discuss and vote on the questions. They end up going around and having each committee member give their thoughts with respect to efficacy and safety. Once this is complete, the committee votes. As the vote was about to happen, Peter Marks, M.D., PhD, the director of the Center for Biologics Evaluation and Research (CBER) at the FDA, turned his camera on and contributed for the first time that day.
One might hope such a powerful figure in the FDA might talk about the importance of such a vote, of course without trying to influence it. He might thank the committee, the sponsor, and his colleagues at the FDA for their work to get to the end of an arduous process. If you watch from 7:55:00, you can see that instead he interjected to request that Pfizer be allowed the last word prior to a vote.
The optics were unseemly, prompting a visceral reaction in my gut. I was literally nauseated by this genuflection to Pfizer.
Their representative went on to make unsubstantiated claims about the effectiveness of their product. He also made light of the outstanding safety concerns and was more than happy to kick the can down the road to “post-approval pharmacovigilance”. More on that later, let’s discuss safety.
Safety
This part will be brief, as there are only a few facts on the ground, and not much to add since my previous post analyzing MATISSE.
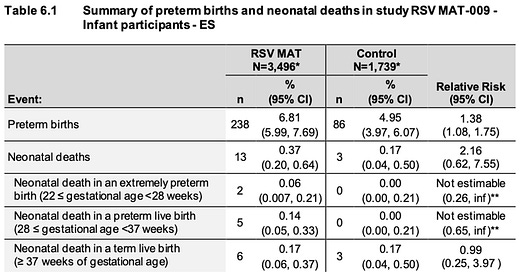
GSK has a nearly identical vaccine, of the same dose, also given to pregnant mothers in the same timeframe (24-36wks gestational age). In February 2022, they halted vaccination throughout their maternal RSV vaccine program after they detected an increased risk of preterm birth and neonatal death in RSV MAT-009:
Pfizer’s MATISSE trial had a directionally higher percentage of preterm births in the vaccinated arm. Preterm births accounted for 4.7% of births in the placebo arm, and 5.7% of births in the vaccine arm. Here is that data:
MATISSE is the only phase 3 trial from Pfizer using this product. Their phase 2b trial, though smaller (~575 total pregnant women), also showed preterm births to be higher in every single arm that received the vaccine, relative to placebo.
If you are like me, you probably see all this data and wonder how anyone could vote in favor of the safety profile of this vaccine. As I listened to the meeting, I can share how ten of the members seemed to get there. In my opinion, they did it by ignoring the question they were asked. As a reminder: they were asked if “available data” were adequate to support the safety of this product.
They all decided they are comfortable relying on post-approval pharmacovigilance data to determine whether this risk was real. I can’t say it’s hard for me to blame them, but I can at least sympathize. Many of the members have seen the burden of RSV in infants up close, and especially with the outsized mental bandwidth RSV currently occupies (given last fall’s surge), many may have personal memories that make them want to see a successful vaccine approved.
Of course, Pfizer did its job and made all the right noises to reassure members that there would be rigorous surveillance that could quickly dispel any concerns about preterm births. I pray that is the case, but recent memory does not reassure me. Think about how difficult and controversial it was to finally come around to acceptance that young men faced a high risk of myocarditis from the second dose of Pfizer and Moderna’s covid shots. Myocarditis in young men is rare. Detecting an increase in a rare signal is child’s play compared to detecting something in the range of a 20% relative risk increase in preterm births, which are common.
By the way, if that relative risk increase holds true, and the real rate of preterm birth is around 10%, as discussed in the meeting, that would lead to roughly a 2% absolute increase in preterm births. I think a “yes” vote with respect to safety based on this data is reckless. I hope they get lucky. Now let’s turn to efficacy, where I was surprised how my intuitions and baseline skepticism compared to the committee members.
Efficacy
Basically, every member of the committee praised the efficacy data from MATISSE. In my mind, the data presented to the committee was uninterpretable. The committee was apparently satisfied to see a decrease in severe RSV-associated LRTIs. While I agree this is promising, how can we have any idea of the potential benefit without looking at all-cause severe LRTI?
If you asked me why an RSV vaccine is desirable for this population, I would have to talk about the burden of RSV. I might say it accounts for a large percentage of lower respiratory tract infections (LRTI) in infants, which can cause bronchiolitis and lead to severe illness, hospitalization, and rarely, (in the US) death.
It naturally follows that if this vaccine is doing what we expect it do to, we should see an overall reduction in LRTI, severe LRTI, LRTI hospitalizations, etc. Pfizer presented zero all-cause data to the VRBPAC. They only gave one all-cause outcome in the MATISSE trial, which we have previously seen, and it showed no benefit whatsoever. As a reminder, here is that data:
One could argue that this data is only MA-LRTI, and we saw a larger benefit with RSV-associated severe MA-LRTI, to which I would readily agree. My qualm: no one required Pfizer to prove any benefit in all-cause severe MA-LRTI. It’s worse than that. Not only were they not required to prove any benefit on all-cause outcomes, they weren’t even required to show us the data. Even if not statistically significant, it should at least show a trend toward better vaccine efficacy.
I can cross my fingers and hope that it directionally improves with severe MA-LRTI, maybe even further improvement with LRTI hospitalization, but I simply have no idea. Are we truly so self-assured in our understanding of this disease process that we don’t even need to prove the product puts a dent in the overall burden? People can argue this study period was a bizarre time for RSV seasonality, RSV caused an unusually low percentage of LRTI, and more. Does that relieve Pfizer of the burden of proving Abrysvo improves the overall burden of LRTI?
We need a stronger FDA
I find myself mostly disappointed in the FDA, and to a lesser extent the VRBPAC. Pfizer’s actions were purely predictable, if detestable at times. As my previous post mentioned, they omitted data in the primary analysis of their publication, biasing the results. They used crude tactics like inconsistent data presentation with respect to scale, and these were shown to be part of a larger strategy during the VRBPAC meeting. In Pfizer’s presentation, they included confidence intervals and made misleading claims when it suited them, and omitted confidence intervals for other results when it would have weakened their argument.
Pfizer consistently conflated the burden of RSV with the presumed effectiveness of their product, which seems to have worked for them in this meeting. They appealed to the committee in terms of the global disease burden, especially in middle- and lower-income countries. They did so in what often felt like the same breath in which they minimized the preterm birth signal because it happened to be concentrated in South Africa, reassuring the committee that high income countries had no serious safety signal. I could go on, but Pfizer acted how we might expect a company to act with billions of dollars on the line. The problem of pharmaceutical companies behaving in their self-interest is solvable, but only if the FDA does its job.
The FDA needs to require far more rigorous reporting of data. At bare minimum they should have required all-cause data for every single outcome they reported with respect to RSV positive cases specifically. In my opinion hospitalization with RSV should have been the primary outcome. The FDA should stop allowing pharmaceutical companies to look at their data periodically and halt their trials if one of the primary outcomes just so happens to look good. The threshold for premature trial termination should be far higher than the threshold we might use for approval. This wasn't a pandemic with a novel virus. There is no excuse for allowing Pfizer to stop enrollment so early with this data. We saw an especially sad demonstration of the consequences of this practice in MATISSE.
Committee members were concerned about the increased rate of preterm births. They asked a statistician roughly what sample size would have been required to detect a 20% increase in preterm births in this population. The answer provided was roughly 10,000. MATISSE was halted early, following their second interim analysis, in which they found one of the two primary outcomes met the predefined success criteria. The study population at that time was 7,358. If they had continued the trial for even another few months, which included the big RSV wave, we would have more confidence is assessing whether the product is safe and/or effective.
As it stands, the FDA required limited safety data, and the minimum sample size should have been increased following the signal of preterm births in the phase 2b trial, as was pointed out by Hana El Sahly, M.D., the VRBPAC Chair.
We were also told that breastfeeding had no impact on vaccine efficacy, but Pfizer did not produce this data during the meeting. This should have been required by the FDA. The status quo is that we allow pharmaceutical companies to design trials, own the data, pay medical writers to write the paper for publication in elite journals, and employ countless tactics to bias the results of studies that are used for regulatory approval. A weak FDA cannot be our only safeguard against such a flawed system.
Final Thoughts
While the FDA has not made their final decision, I expect this vaccine will be approved. If that happens, we will be rolling the dice with respect to preterm births. Pfizer clearly has every reason not to find an increased rate of preterm births following theoretical approval. The FDA would have to require rigorous, large studies to detect this potential safety signal. I doubt if we will ever really know whether this vaccine is a net positive or negative. I hope we get lucky, and it is the former.